
Evidence on the Carcinogenicity of
Bisphenol A
Carcinogen Identification Committee Meeting
December 14, 2022
Cancer Toxicology and Epidemiology Section
Reproductive and Cancer Hazard Assessment Branch
Office of Environmental Health Hazard Assessment, CalEPA

Overview
• Introduction
• Carcinogenicity data on Bisphenol A (BPA)
• Epidemiologic studies
• Animal studies
• Mechanistic data
• Pharmacokinetics and metabolism
• Key characteristics (KCs) of carcinogens
2

Bisphenol A (BPA)
• C
15
H
16
O
2
(CAS: 80-05-7) • High production volume chemical
with many applications, including
• polycarbonate plastics
• epoxy resins
• Ubiquitous presence in the
environment
• Exposure pathways: contaminated
food and water, ingestion of dust,
inhalation, dermal contact, in utero
transfer, lactation
3

Human Exposure to BPA
• Widespread human exposure across all life stages
• Biomonitoring studies find BPA in urine, serum, and tissue
in the majority of individuals
• Decreases in BPA detection frequency and levels in recent
years in general population samples
• Measurements of BPA through biomonitoring:
• Reflect short-term exposure (half-life ~6 hours)
• May not capture high variation in exposure levels
4

5
Epidemiologic
evidence
Multiple studies on
breast, prostate, and
thyroid cancers
Evidence from animal studies
Multiple study designs
Pharmacokinetics and metabolism
Key characteristics of carcinogens

Epidemiologic Studies
6

7
• 51 records identified
• Included all analytical studies, with
consideration of:
• Study quality
• Direction and magnitude of biases
• Hill guidance for body of evidence
• Excluded conference abstracts,
reviews, studies on uterine
leiomyoma
Epidemiologic Studies - Overview
Cancer Site
Studies
Breast 13
Prostate 3
Thyroid 2
Lung 2
Bile duct/gallbladder 1
Bone 1
Brain 1
Endometrium 1
Eye 1
Lymphohematopoietic system 1
All cancer mortality 1

Epidemiologic Studies – Key Issues
• BPA measurement/estimation: long-term exposure may not be represented
• Measurement error could bias risk estimates towards or away from the null
• Single time point BPA measurement does not account for highly variable levels
• Limitation of all the biomonitoring studies
• Cumulative BPA estimation is also limited
• Questionnaires: poor correlation with urinary BPA levels
• Job Exposure Matrices: do not capture widespread exposure from non-occupational sources
• Timing of sample collection: at/after diagnosis
• Relevant time window not assessed – true causal effects could be missed
• Reverse causation could not be ruled out
• In cross-sectional studies with cancer outcomes: prevalent cancers may reflect survivor
bias, temporality not established
8

9
BPA and Breast Cancer

10
BPA and Thyroid, Prostate Cancer
Tumor
site
Reference Study
Design
Exposure Assessment
Method
Exposure category
or level
RR (95% CI)
Thyroid
Marotta et al.
(2019)
Cross-sectional
Serum BPA measured using
HPLC/FLD/UV
Exposed to BPA 3.71 (0.67–20.34)
Zhou et al.
(2017)
Cross-sectional
Urinary total BPA measured
using HPLC–MS/MS
Urinary BPA >2.84 ng/ml
(not adjusted for
creatinine)
3.57 (1.37–9.3)
Prostate
Salamanca-
Fernández et
al. (2021)
Case-cohort
Serum BPA analyzed by
DLLME & UHPLC-MS/MS
Tertile 3 (5.1–68.9 ng/ml
BPA)
1.31 (0.98–1.74)
Tse et al.
(2017)
Case-control
Cumulative BPA index from
questionnaire data and lit
review
High Cumulative BPA
Index
1.88 (1.24–2.86)

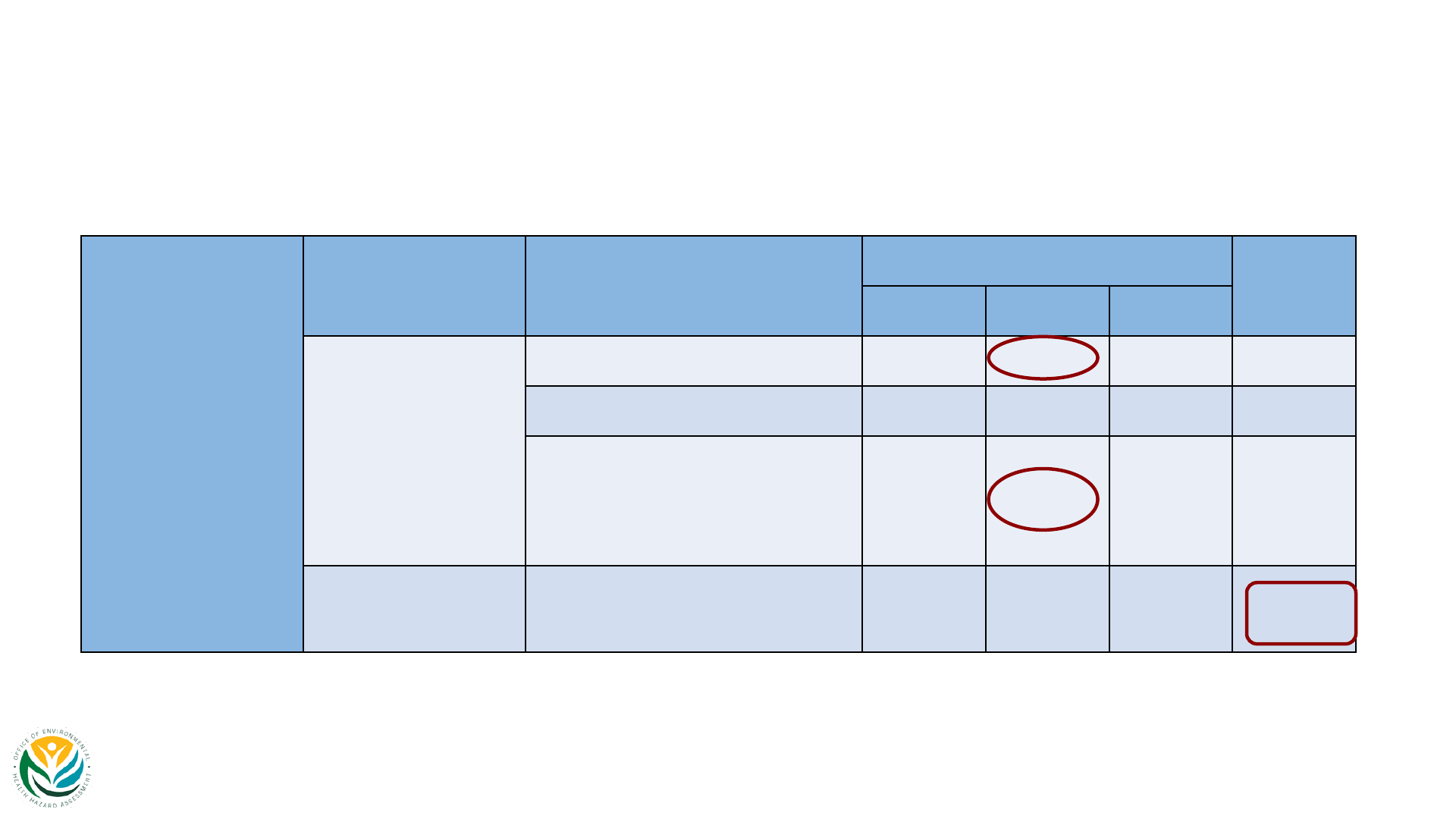
Carcinogenicity Studies in Animals
11

Animal Carcinogenicity Studies – Overview
12
Exposure Species Strains Sex (# of studies) Route Study duration
Beginning
at or after
four weeks
of age
Mouse B6C3F1 male (1), female (1) feed 107 weeks
Rat F344 male (1), female (3) feed, gavage, 12-108 weeks
Gerbil Not specified male (2) drinking water 24-29 weeks
In utero or
within the
first week
of life
Mouse
CD-1, Agouti
+/–
C57BL/6J:C3H/
HeJ
male (1), female (4)
s.c., in utero, in utero
and via lactation &
feed
3-18 months
Rat
SD (NCTR),
F344, SD,
Wistar-Furth
male (5), female (7)
In utero, in utero and
gavage, in utero and
via lactation
PND50 up to 2
years
Exposure Species Strains Sex (# of studies) Route Study duration
Beginning
at or after
four weeks
of age
Mouse B6C3F1 male (1), female (1) feed 107 weeks
Rat F344 male (1), female (3) feed, gavage, 12-108 weeks
Gerbil Not specified male (2) drinking water 24-29 weeks
In utero or
within the
first week
of life
Mouse
CD-1, Agouti
+/–
C57BL/6J:C3H/
HeJ
male (1), female (4)
s.c., in utero, in utero
and via lactation &
feed
3-18 months
Rat
SD (NCTR),
F344, SD,
Wistar-Furth
male (5), female (7)
In utero, in utero and
gavage, in utero and
via lactation
PND50 up to 2
years

Assessing dose-response significance
• Statistical tests are performed using effective number when possible
• One-sided Fisher’s exact test for pairwise comparisons
• Exact conditional Cochran-Armitage test for linear trend
• The test originally derived by Cochran and Armitage relies on a Normal
approximation
• Performs well for large and balanced sample sizes
• Williams (1988) demonstrated that using the exact conditional distribution of the
test statistic improves the accuracy of the test
• The algorithm used to derive the exact p-value is described in Mehta et al (1992)
Williams DA (1988). Tests for differences between several small proportions. Journal of the Royal Statistical Society.
Series C [Applied Statistics] 37(3): 421-434.
Mehta CR, Patel N, and Senchaudhuri P (1992). Exact Stratified Linear Rank Tests for Ordered Categorical and Binary
Data. Journal of Computational and Graphical Statistics 1(1):21–40.
13
14
103-week feed
study in male
B6C3F1 mice
(NTP 1982)
Site Type
Concentration in feed (ppm)
Exact
trend test
p-value
0 1000 5000
Hematopoietic
system
Malignant lymphoma 2/47 8/47* 3/45 NS
All leukemia [rare] 0/44 1/46 2/45 NS
Malignant lymphoma and
lymphocytic leukemia
combined
2/47 9/47* 3/45 NS
Pituitary
Chromophobe carcinoma
[rare]
0/37 0/36 3/42 0.0465
Tumor Findings in Male Mice:
Exposed to BPA Beginning at or after Four Weeks of Age
* p < 0.05; NS, not significant

15
103-week
feed study in
male F344 rats
(NTP 1982)
Site Type
Concentration in feed (ppm)
Exact
trend test
p-value
0 1000 2000
Hematopoietic
system
Leukemia (NOS) 13/50 12/50 23/50* 0.021
Mammary gland Fibroadenoma 0/36 0/40 4/34* 0.008
Testis
Interstitial (Leydig) cell
tumor
35/47 48/48*** 46/49** 0.0015
12-week
oral study in
female F344 rats
(Hao et al. 2016)
Site Type
Concentration (mg/kg-day)
Exact
trend test
p-value
0 50 200 400
Pituitary gland Pituitary tumor 0/10 4/10* 1/10 3/10 NS
Tumor Findings in Rats:
Exposed to BPA Beginning at or after Four Weeks of Age
* p < 0.05; ** p < 0.01; *** p < 0.001; NS, not significant; NOS, not otherwise specified

Female F1
Agouti
+/–
C57BL/6J:C3H/HeJ
mice
(Weinhouse et al.
2014)
Site Type
Concentration (ppm)
Exact
trend
test p-
value
0 5×10
–5
0.05 50
Liver
Hepatocellular carcinoma 0/9 2/10 1/10 3/9 NS
Combined hepatocellular
adenoma or carcinoma
0/9 2/10 1/10 4/9* 0.0185
16
Tumor Findings in Female Mice:
Exposed to BPA Beginning
in utero
, via Lactation,
and Post-weaning in Feed Until 10 Months of Age
* p < 0.05; NS, not significant

#1
#2
#3
#4
#5
#6
#7
#8
Gavage daily to dam
Gavage daily
Dose: 0 (vehicle); BPA at 2.5,
25, 250, 2500 or 25000
µg/kg per day
No treatment
Overview of the CLARITY-BPA Core Studies Conducted in SD (NCTR) Rats
17

18
Study Tumor site
Tumor
type
Dose
(µg/kg-day)
Exact trend
test p-value
0 2.5 25 250 2500 25000
Stop-dose
(in utero +
3 weeks)
2-year (#3)
Mammary
gland
Adenoma 1/48 1/44 0/43 3/45 0/47 1/40 NS
Adeno-
carcinoma
3/48 11/44* 5/45 7/48 9/47 5/41 NS
Combined 4/48 12/44* 5/45 9/48 9/47 6/41 NS
Continuous
-dose 1-
year (#5)
Uterine
Stromal
polyps
1/20 0/20 1/21 0/22 3/20 3/24 p < 0.05
Continuous
-dose 2-
year (#7)
Clitoral
gland
Adenoma 0/40 0/38 0/32 0/41 0/33 2/36 p < 0.05
Carcinoma 1/50 1/44 1/43 1/47 4/47 1/45 NS
Combined 1/50 1/44 1/43 1/47
4/47 3/45 p < 0.05
* p < 0.05; NS, not significant
CLARITY-BPA Core Studies in SD (NCTR) Rats
Tumor Incidences in Females

19
Study Tumor site Tumor type
Dose
(µg/kg-day)
Exact trend
test p-value
0 2.5 25 250 2500 25000
Stop-dose
(in utero +
3 weeks)
2-year (#4)
Prostate
(dorsal/later
al lobes)
Malignant
lymphoma
0/49 0/48 0/48 3/50 2/49 4/45* p < 0.01
All sites
Malignant
lymphoma
1/49 0/48 1/48 3/50 2/49 5/45 p < 0.01
Thyroid
gland
C-cell
adenoma
0/37 1/31 0/33 0/26 1/34 3/23 p < 0.05
Continuous
-dose 2-
year (#8)
Liver
Hepatocellular
carcinoma
[Rare]
0/24 0/25 0/24 2/24 1/24 3/19 p < 0.01
* p < 0.05
Tumor Incidences in Males
CLARITY-BPA Core Studies in SD (NCTR) Rats

Evaluation of Rare Tumors in SD (NCTR) Rats
• Rare tumors were observed
• SD (NCTR) rats were on CLARITY-BPA core study from 2012 to 2015
• Lack of ideal historical control data
• 3 databases used
• NTP (2008, 2010) (dietary/feed administration, SD (NCTR) rats, 1999 to 2003)
• Charles River (2013) (oral routes, Crl:CD®(SD)BR rats, 2001 to 2009)
• NTP (2021) (all routes, Hsd SD rats, 2007 to 2012)
• Each of the 3 databases with its own unique set of limitations
• Rare tumors presented in the HID
• Less than 1% of tumor incidence in historical control animals in each of the three sets
of historical control data
• With no tumor occurrence in the concurrent controls
20

Additional Issues Associated with the CLARITY-BPA
Core Studies
• Possible exposure of controls to BPA via contamination
• BPA levels in vehicle and naive control animals were similar to the levels
detected in the lowest BPA exposure group
• Insensitive responsiveness of the SD (NCTR) rats
• Insensitive to known estrogens, such as ethyl estradiol (EE2)
• Insensitive to known thyroid peroxidase inhibitor, 6-propyl-2-thiouracil (PTU)
• Additional concerns
• Lack of an unhandled, non-gavaged control group and lack of EE2-treated
positive controls in the stop-dose arms
21

Tumor Findings: By System and Tumor Type
• Alimentary system: Hepatocellular tumors in male SD (NCTR) rats, and female
Agouti
+/–
C57BL/6J:C3H/HeJ mice
• Endocrine system: Pituitary tumors in female F344 rats and male B6C3F1
mice; thyroid C-cell tumors in male SD (NCTR) rats
• Mammary gland
: Fibroadenoma in male F344 rats; adenocarcinoma, and
adenoma and adenocarcinoma combined in female SD (NCTR) rats
• Reproductive system
:
Female: Clitoral gland tumors & uterine stromal polyps in SD (NCTR) rats
Male: Testicular interstitial (Leydig) cell tumors in F344 rats
• Lymphohematopoietic system
: Leukemia in male F344 rats, lymphoma in
male SD (NCTR) rats and male B6C3F1 mice
• Multiple types of r
a
re tumors were observed in several studies in male and
female SD (NCTR) rats.
22

Tumor Findings from Transgenic Animal models
• Female mouse (MMTV-erbB2) mammary tumor models
• ↓ tumor latency in two studies
• ↑ tumor multiplicity
• ↑ tumor volume
• ↑ lung metastases of mammary tumors
• Mouse model with an estradiol non-responsive mutant ER-α
ligand binding domain
• ↑ “tumor-like outgrowths” (adenocarcinomas) in the flank muscle of
female transgenic mice
23

Tumor Findings from Other Animal Models
• In xenograft, syngeneic, and regenerated organ mouse models
• ↑ No. of tumor-bearing mice, mean tumor volume or tumor weight in xenograft
models (BPA, xenograft)
• ↑ g
rowth of established tumors in xenograft models (xenograft, BPA)
• ↑
tumor volume in syngeneic mouse models
• ↑ atypical ductal hyperplasia and ductal carcinoma in sit
u i
n regenerated mammary
glands
• BPA in combination with other treatments
• ↑ mammary tumor incidence and /or multiplicity in female rats, ↓ tumor latency in
female rats and mice (BPA, carcinogen)
• ↑ mammary tumors in female rats (tumor initiator, BPA)
• ↑ microinvasive carcinoma and PINs of the prostate in male rats (BPA, testosterone &
1
7β-estradiol)
24

Break for Clarifying Questions from the
Carcinogen Identification Committee
CIC Meeting - December 14, 2022
25

Mechanistic considerations and
other relevant data
26

Pharmacokinetics and Metabolism
• BPA is rapidly absorbed and widely distributed in humans
• Crosses blood-brain barrier and placenta
• Detected in breastmilk, adipose tissues, liver and other organs and body fluids
• Half-lives vary by species and administration route (< 24 hours)
• Humans by oral route: ~ 6 hours
• In humans, fast excretion mainly via urine (detected in more than
90% of NHANES population)
• Feces as the main route of excretion in rodents
• Enterohepatic circulation in rodents, not humans
27

28
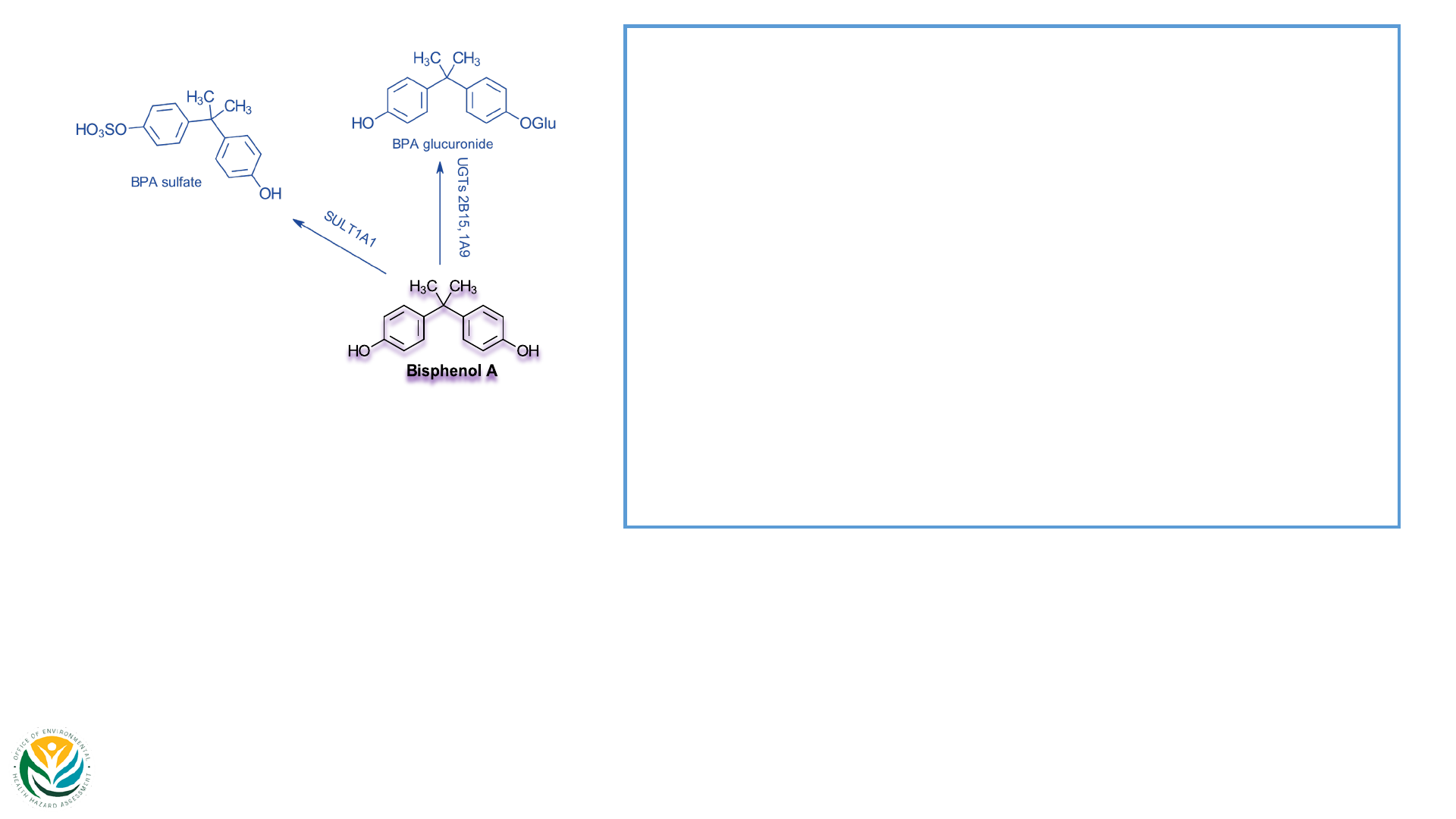
29 view 1
Phase II metabolism:
• Glucuronidation:
• BPA-G; Primary enzymes include UGT2B15 &
UGT1A9
• ~70% of excreted metabolites (main metabolite in
humans and animals)
• Crosses placenta; subsequent de-conjugation of
BPA-G to BPA by fetal β-glucuronidases
• Sulfoconjugation:
• BPA-S; Primary enzymes include SULT1A1
• ~20% of excreted metabolites
• De-conjugation via sulfatases (estrone sulfatase)

29 view 2
Factors affecting conjugation (section 5.1.4):
• Genetic polymorphisms: e.g., UGT2B15*2 leads to significantly
decreased glucuronidation
• De-conjugation reactions: Estrone sulfatase, fetal β-glucuronidases
• Co-exposures to xenobiotics & medications (naproxen,
carbamazepine)
• Disease status: Reduced glucuronidation (Parkinson’s) and sulfation
(liver disease; up to 80% reduction)
• Life stage: UGT1A1 absent from the fetal liver; UGT2B15 active at
reduced levels in human fetus

29 view 3
[1] ortho-OH-BPA: estrogenic activity;
induces proliferation of human breast
cancer cells
Biologically active and reactive
metabolites are shown in red color

29 view 4
Biologically active and reactive
metabolites are shown in red color
[2] BPA-3,4-quinone: DNA
adducts; ROS formation and
oxidative stress

29 view 5
[3] Arene epoxide intermediate:
reactive metabolite
Biologically active and reactive
metabolites are shown in red color

29 view 6
Biologically active and reactive
metabolites are shown in red color
[4] Carbocation intermediate:
reactive metabolite; forms
IPP & HCA
[5] HCA:
estrogenic
activity

29 view 7
Biologically active and reactive
metabolites are shown in red color
[6] Radical intermediate: IPP
and its intermediate radical
from MBP

29 view 8
Biologically active and reactive
metabolites are shown in red color
[7] MBP: estrogenic activity; induces
proliferation of human breast cancer cells

29 view 9
Biologically active and reactive
metabolites are shown in red color

30
Key Characteristics
of Carcinogens
Images of the KCs are adapted from
Guyton et al. (2018) & Smith et al.
(2020) with modifications. See also
Preamble to the IARC monographs
(IARC 2019).

KC 8: Modulates Receptor-mediated Effects
Effects on Estrogen Receptors (ERs)
• Classical ER-mediated effects, e.g. in vitro and in vivo estrogenicity (Chapin et
al. 2008)
• BPA modulates ER-m
e
diated effects through several ER subtypes
• Non-c
anonical ER activities, e.g. the
rapid onset of extranuclear responses,
the low-dose effects and the non-monotonic dose-responses (↑ female rat
mammary tumor in CLARITY-BPA core study #3)
• For example, BPA affects membrane-associated estrogen receptors, G-
protein coupled estrogen receptor, and estrogen-related receptor gamma
• BPA can induce epigenetic changes to regulate the expression of ERα a
nd
c
ancer-related ER target genes
31
Section 5.3.8 and Appendix J

Effects on progesterone receptor
• ↑ PR expression in human and mammalian in vitro studies
Effects on androgen receptor
• Exhibited antiandrogenic activity in human and mammalian in vitro studies
Effects on thyroid hormone receptors
• Antagonized TRβ activity in human in vitro studies
Effects on other nuclear receptors
• Altered expression or activity of PPARα, PPARγ, AhR, and PXR
KC 8: Modulates Receptor-mediated Effects (cont ’d)
Section 5.3.8 and Appendix J
32

Effects on hormone levels
• Estradiol: positive correlations in some human observational studies in
specific populations
• Testosterone: positive association in women and girls with PCOS
• ↓ testosterone levels in male mice
• Prolactin: positive associations in human occupational studies
• ↑ prolactin levels in rats
• No consistent associations with progesterone or thyroid hormones
KC 8: Modulates Receptor-mediated Effects (cont’d)
Section 5.3.8 and Appendix J
33

KC 10: Alters Cell Proliferation, Cell Death,
or Nutrient Supply
• ↑ cell proliferation in human cell lines (normal, immortalized, and cancer cells) in
vitro
• ↑ hyperplasia and cell proliferation in multiple organs in multiple strains of rats
a
nd m
ice in vivo
• ↓ apoptosis, ↑ anti-a
pop
totic proteins (e.g. Bcl-2), ↓ pro-apoptotic proteins
(e.g. BAX, caspases) in human cancer cell lines
• Altered signaling pathways related to cell cycle control (e.g. ↑ cyclins, CDKs and
PCNA, ↓ p21 and p53) in human cancer cell lines
• ↑ angiogenesis in human HUVEC cells and ↑ pro-a
ng
iogenesis gene expression
(e.g. VEGF) in human cells (normal and cancer)
• ↑ glycolysis-ba
s
ed energy production in human cancer cell lines
34
Section 5.3.10 and Appendix K

KC 1: Is Electrophilic or Can Be Metabolically
Activated
• Multiple electrophilic and reactive metabolites
• BPA-3,4-quinone (BPAQ)
• Arene epoxide intermediate
• IPP radical, which forms MBP
• Unidentified electrophilic compound leading to BPA dimer
• Oxidative lesions in DNA (8-hydroxydeoxyguanosine; 8-OHdG) (KC2, KC5)
• Formation of DNA adducts in vivo and in vitro, and cell-free systems
• Binds to cysteine residues to form protein adducts (rat in vivo and in a
cell-free system)
35
Section 5.3.1

KC 2: Is Genotoxic
Mutations
• ↑ in human embryo-derived fibroblasts and HEK 293T cells in vitro
• ↑ in dominant lethal mutation rate in male rats in vivo
• No effects in bacteria, yeast, or Drosophila
Chromosomal effects
• ↑ in MN, CA, and various types of chromosomal abnormalities in in vitro
studies (human and animal cells) and in vivo animal studies
• ↑ in CA in plants in 3 studies; ↑ in microtubule abnormalities in acellular
systems in 2 studies
36
Section 5.3.2 and Appendix F

KC 2: Is Genotoxic (cont’d)
DNA damage
• Positive associations between urinary or serum levels of BPA and 8-
OHdG (> 10 human observational studies)
• Positive associations between urinary BPA levels and sperm DNA
fragmentation (2 human observational studies)
• ↑ DNA adduct formation, DNA strand breaks, oxidative damage to DNA,
and γ-H2AX in multiple experimental systems
• ↑ DNA damage-control protein expression in 2 types of human cells in
vitro and in an earthworm in vivo study
37
Section 5.3.2 and Appendix F

KC 5: Induces Oxidative Stress
• ↑ oxidative damage to DNA (8-OHdG)
• 13 of 19 human observational studies
• 3 of 3 rodent in v
iv
o studies
• ↑ reactive oxygen or nitrogen species in more than 100 human in
vitro and rodent in vivo and in vitro studies
• Dose- or concentration-dependent increases in some studies
• ↑ lipid peroxidation (8-isopropane or malondialdehyde) in human
observational studies, human in vitro and rodent in vivo studies
• ↓ GSH or antioxidant enzyme activities or levels in rodent in vivo
and in vitro studies
38
Section 5.3.5 and Appendix H

KC 3: Alters DNA Repair or Causes Genomic Instability
DNA repair capacity
• ↓ repair of DNA damage in human cells in vitro and rodent cells in vitro
DNA repair genes
• ↓ MyH, TP53 expression in human cells in vitro
• ↓ mlh1 expression in Drosophila melanogaster (1 study)
39
Section 5.3.3

40
KC 4: Induces Epigenetic Alterations
Epigenetic findings in human observational studies and human
cells in vitro, as well as animals in vivo and animal cells in vitro
Altered methylation of regions associated with specific genes
• E.g., promoter hypermethylation of CAPS2 and TNFRSF25 in human cord blood
Global methylation changes
• E.g., LINE-1 methylation in human cord blood
miRNA changes
• E.g., altered expression of cancer-related miRNAs in human cells in vitro
Histone modifications
• E.g., altered regulation of mRNA expression of HDACs and HATs in human cells
in vitro
Section 5.3.4 and Appendix G

KC 6: Induces Chronic Inflammation
Human observational studies
• Positive associations with C-reactive protein (CRP) and tumor necrosis factor-α
(TNF-α) (8 cross-sectional studies)
• Positive association with interleukin-6
(
IL-6) (8 cross-sectional, 1 cohort study)
• No significant association with IL-1
β,
IL-10, TNF-α, or CRP (2 cohort studies)
Animal studies
• Chronic inflammation with longer-term BPA exposure (many studies)
• Histopathology in many tissues
• Significant increases in pro-in
f
lammatory biomarkers including IL-1β, IL-6,
TNF-α
• Negative association between BPA exposure and inflammatory biomarkers (2
s
tudi
es)
41
Section 5.3.6 and Appendix I

KC 7: Is Immunosuppressive
• Effects on T cell and B cell cellularity or proliferation
• ↓ T and B cell cellularity or proliferation in human cells in vitro, rodents
in vivo, rodents in vitro, and fish
• Effects on neutrophils
• ↓ chemotactic capacity in human cells in vitro and mice
• Effects on macrophages
• ↓ phagocytosis in human cells in vitro, rats, mice, and fish
• ↓ macrophage populations in mice (1 study)
• ↓ macrophage proliferation in fish (1 study)
42
Section 5.3.7

• Effects on dendritic cells
• ↓ endocytotic capacity in human cells in vitro (1 study)
• ↓ dendritic cells in rats (1 study)
• Effects on natural killer cells
• ↓ percentage of splenocytes that were NK cells in mice (1 study)
• Effects on IgM levels
• ↓ IgM levels in mice and fish
KC 7: Is Immunosuppressive (cont’d)
43
Section 5.3.7

KC 9: Causes Immortalization
• Cell transformation
• ↑ transformation frequency in Syrian hamster embryo cells
• Cell invasion
• ↑ in Matrigel invasion assays of multiple types of primary human cells in vitro
• Epithelial-Mesenchymal-Transition markers
• ↑ vimentin, fibronectin, snail, and MMP-9 expression in human cells in vitro
• ↓ E-cadherin expression in human cells in vitro
• No change in slug expression in human cells in vitro
• Cellular senescence markers
• ↓ p21 expression in human cells in vitro (1 study)
• Telomerase expression, activity, or telomere length
• ↓ telomere length in women (1 study)
• Altered telomerase expression or activity in human cells in vitro
44
Section 5.3.9

45 view 1
Key Characteristics
of Carcinogens:
BPA
KC1
• Electrophilic metabolites
• DNA & protein adducts
• Oxidative lesions in DNA
Images of the KCs are adapted from Guyton et al.
(2018) & Smith et al. (2020) with modifications.
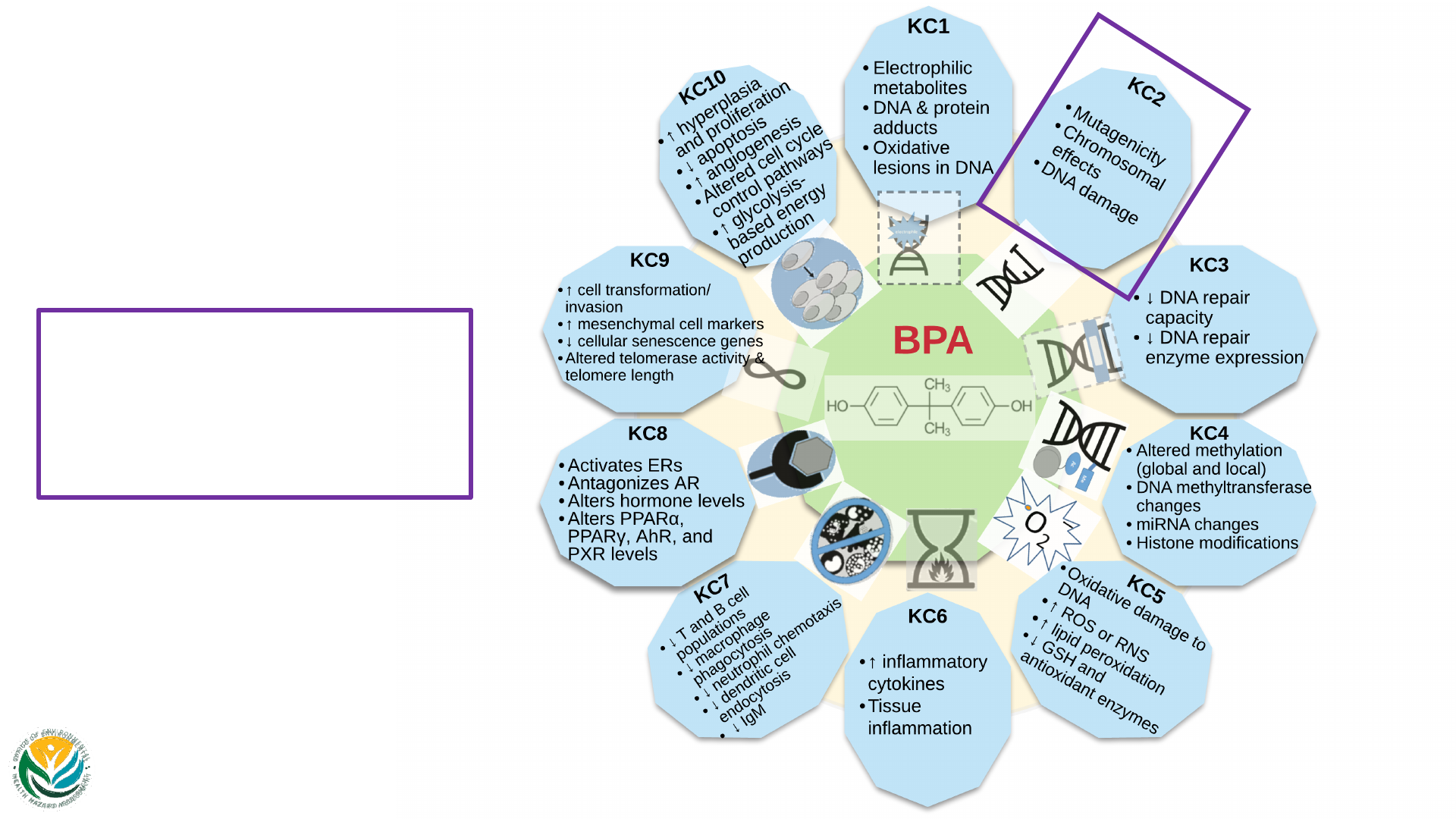
45 view 2
Key Characteristics
of Carcinogens:
BPA
KC2
• Mutagenicity
• Chromosomal effects
• DNA damage
Images of the KCs are adapted from Guyton et al.
(2018) & Smith et al. (2020) with modifications.

45 view 3
Key Characteristics
of Carcinogens:
BPA
KC3
• ↓ DNA repair capacity
• ↓ DNA repair enzyme
expression
Images of the KCs are adapted from Guyton et al.
(2018) & Smith et al. (2020) with modifications.

45 view 4
Key Characteristics
of Carcinogens:
BPA
KC4
• Altered methylation
(global and local)
• DNA methyltransferase
changes
• miRNA changes
• Histone modifications
Images of the KCs are adapted from Guyton et al.
(2018) & Smith et al. (2020) with modifications.

45 view 5
Key Characteristics
of Carcinogens:
BPA
KC5
• Oxidative damage to
DNA
• ↑ ROS or RNS
• ↑ lipid peroxidation
• ↓ GSH and antioxidant
enzymes
Images of the KCs are adapted from Guyton et al.
(2018) & Smith et al. (2020) with modifications.

45 view 6
Key Characteristics
of Carcinogens:
BPA
KC6
• ↑ inflammatory
cytokines
• Tissue inflammation
Images of the KCs are adapted from Guyton et al.
(2018) & Smith et al. (2020) with modifications.

45 view 7
Key Characteristics
of Carcinogens:
BPA
KC7
• ↓ T and B cell populations
• ↓ macrophage phagocytosis
• ↓ neutrophil chemotaxis
• ↓ dendritic cell endocytosis
• ↓ IgM
Images of the KCs are adapted from Guyton et al.
(2018) & Smith et al. (2020) with modifications.

45 view 8
Key Characteristics
of Carcinogens:
BPA
KC8
• Activates ERs
• Antagonizes AR
• Alters hormone levels
• Alters PPARα, PPARγ,
AhR, and PXR levels
Images of the KCs are adapted from Guyton et al.
(2018) & Smith et al. (2020) with modifications.

45 view 9
Key Characteristics
of Carcinogens:
BPA
KC9
• ↑ cell transformation/
invasion
• ↑ mesenchymal cell
markers
• ↓ cellular senescence genes
• Altered telomerase activity
& telomere length
Images of the KCs are adapted from Guyton et al.
(2018) & Smith et al. (2020) with modifications.

45 view 10
Key Characteristics
of Carcinogens:
BPA
Images of the KCs are adapted from Guyton et al.
(2018) & Smith et al. (2020) with modifications.
KC10
• ↑ hyperplasia and
proliferation
• ↓ apoptosis
• ↑ angiogenesis
• Altered cell cycle control
pathways
• ↑ glycolysis-based energy
production
