
Open camera or QR reader and
scan code to access this article
and other resources online.
Analysis of Growth Velocity in Children with Attention-Deficit/
Hyperactivity Disorder Treated for up to 12 Months
with Serdexmethylphenidate/Dexmethylphenidate
Ann C. Childress, MD,
1
Andrew J. Cutler, MD,
2,3
Maitrey Patel, RPh,
4
and Charles Oh, MD
4
Abstract
Objective: Serdexmethylphenidate/dexmethylphenidate (SDX/d-MPH) is approved for the treatment of patients aged
‡6 years with attention-deficit/hyperactivity disorder (ADHD). A 12-month, open-label safety study with SDX/d-MPH in
children with ADHD showed that SDX/d-MPH was well tolerated and comparable with other methylphenidate products. In
this post hoc analysis of the 12-month study, the objective was to characterize the effect of SDX/d-MPH on growth in children
with ADHD over 12 months.
Methods: This was a post hoc analysis of a dose-optimized, open-label, phase 3 safety study of SDX/d-MPH in children aged
6–12 years with ADHD (NCT03460652). Weight and height Z-score analyses were conducted. Z-score change from baseline
was calculated based on the baseline values for the subjects remaining in the study at the observation time point.
Results: Subjects (N = 238) from the treatment-phase safety population included all enrolled subjects who received ‡1doseof
study drug and had ‡1 postdose safety assessment. During treatment, the mean weight and height Z-scores decreased over time from
their respective baselines. At the 12-month time point, mean (standard deviation [SD]) Z-score changes from baseline for weight
and height for the subjects remaining in the study were -0.20 (0.50) and -0.21 (0.39), respectively; however, these mean changes in
Z-scores were not clinically significant (change <0.5 SD). Long-term treatment with SDX/d-MPH was associated with modest
reductions in expected weight and lower-than-expected increases in height: effects that plateaued or diminished later in treatment.
Conclusion: The overall effects of SDX/d-MPH on growth velocity (the change in weight and height from one time point to
the next) were minimal, and the range of changes was not considered clinically significant. ClinicalTrials.gov identifier:
NCT03460652.
Keywords: ADHD, Azstarys, SDX/d-MPH, growth velocity, Z -score
Introduction
M
ethylphenidate (MPH) is a commonly prescribed medi-
cation for attention-deficit/hyperactivity disorder (ADHD)
because of its efficacy for symptom reduction and overall favorable
safety record. MPH is often prescribed for long-term use in patients
with ADHD (Storebø et al. 2018; Krinzinger et al. 2019). Serdex-
methylphenidate (SDX) is a prodrug of dexmethylphenidate (d-
MPH) and is coformulated with immediate release d-MPH in fixed
molar dose ratios of 70% SDX:30% immediate release d-MPH
hydrochloride (HCl).
This formulation of SDX/d-MPH (Azstarys; Corium, LLC,
Boston, MA) was approved by the US Food and Drug Adminis-
tration for patients aged 6 years and older with ADHD.
1
Center for Psychiatry and Behavioral Medicine, Las Vegas, Nevada, USA.
2
SUNY Upstate Medical University, Syracuse, New York, USA.
3
Neuroscience Education Institute, Lakewood Ranch, Florida, USA.
4
Corium, LLC, Boston, Massachusetts, USA.
Funding: Clinical research was funded by Zevra Therapeutics (previously KemPharm, Inc.). Funding for editorial and writing assistance in the form of
proofreading, copyediting, and fact-checking was provided by Corium, LLC.
Data contained in this article were previously presented at the Annual Meeting of the American Academy of Child and Adolescent Psychiatry
(AACAP) Virtual Conference, October 19–24, 2020.
ª Ann C. Childress et al. 2023; Published by Mary Ann Liebert, Inc. This Open Access article is distributed under the terms of the Creative Commons
License [CC-BY] (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided
the original work is properly cited.
JOURNAL OF CHILD AND ADOLESCENT PSYCHOPHARMACOLOGY
Volume 33, Number 4, 2023
Mary Ann Liebert, Inc.
Pp. 134–142
DOI: 10.1089/cap.2023.0012
134

In a pivotal, randomized, placebo-controlled, double-blind lab-
oratory classroom study of children with ADHD, SDX/d-MPH
treatment was shown to significantly improve ADHD symptoms
compared with placebo (Kollins et al. 2021). SDX/d-MPH onset
began by 30 minutes postdose, and the treatment effect lasted up to
13 hours. Adverse events (AEs) were similar to other stimulants
used for the treatment of patients with ADHD.
A subsequent, 1-year, open-label safety study of SDX/d-MPH in
children with ADHD showed that SDX/d-MPH was well tolerated
and had sustained efficacy during the 1-year treatment period
(Childress et al. 2023). Of the 238 subjects assessed in the 1-year
study, the most common treatment-emergent AEs were decreased
appetite (18.5%), upper respiratory tract infection (9.7%), naso-
pharyngitis (8.0%), decreased weight (7.6%), and irritability
(6.7%).
Long-term treatment with stimulants, such as MPH for ADHD,
in children has been associated with modest slowing of growth
velocity and prescribing information for these treatments warns of
long-term growth suppression, which can cause concern and limit
their use (Spencer et al. 2006; Faraone and Giefer 2007; Krinzinger
et al. 2019). The objective of the current investigation was to
characterize the effects of SDX/d-MPH on growth in children with
ADHD treated with SDX/d-MPH from the 1 year, open-label,
safety study (Childress et al. 2023).
Methods
This post hoc analysis of a dose-optimized, open-label, phase 3
safety study of SDX/d-MPH in children aged 6–12 years with
ADHD (NCT03460652) (Childress et al. 2023) was conducted at
18 sites in the United States. The original study protocol and
amendments were approved by an institutional review board before
each center initiated the study. The first patient was screened on
February 21, 2018, and the last follow-up visit was on June 27, 2019.
Subjects
The subjects were children aged 6–12 years with ADHD at the
start of the dose-optimization (DO) phase of the study. Subjects
included those rolled over from the antecedent pivotal study
(Kollins et al. 2021) within 45 days of their last dose of SDX/d-
MPH, and new subjects who did not participate in the pivotal study
or who entered the trial more than 45 days after their last dose in the
pivotal study (Childress et al. 2023). Subjects had to have a body
weight of at least 21 kg at the screening phase and be in good health.
At least one parent or legal guardian was required to provide written
permission, and each subject had to give written or verbal per-
mission for study participation.
Study design
The study design included a 30-day screening phase, a 3-week
DO phase, a 360-day treatment phase, and a follow-up visit. Only
new subjects underwent screening and DO. During the DO phase,
new subjects were titrated to their optimized dose based on the best
dose response and individual tolerability to the treatment (Childress
et al. 2023).
New subjects started treatment with the 39.2/7.8 mg dose of
SDX/d-MPH daily, which is a molar equivalent to 30 mg of to-
tal d-MPH HCl, for 7 days. If needed, dose adjustments were
performed based on the investigator’s assessment of the subject
at weekly intervals. The SDX/d-MPH dose could be increased
to 52.3/10.4 mg (40 mg molar equivalent of total d-MPH HCl),
decreased to 26.1/5.2 mg (20 mg molar equivalent of total d-MPH
HCl), or maintained at the same dose for the next week of dosing.
The dose at the end of the third week was assigned as the opti-
mized dose. Subjects who rolled over from the pivotal study re-
mained on their optimized dose from that study. The optimized SDX/
d-MPH dose was used during the treatment phase. All subjects were
administered 1 capsule daily of their optimized dose of SDX/d-MPH.
Weight and height analyses were conducted in the treatment phase
safety population of enrolled subjects. This population included
those who received at least 1 dose of study medication and had at
least 1 postdose safety assessment in the treatment phase. Com-
pleters are those subjects that completed 12 months of treatment.
Weight and height measurements
Body weight (in kg) and height (in cm) were measured at each
monthly visit. Body weight was measured using a calibrated scale
while subjects remained in their normal clothing, with shoes and
jacket (and/or outer clothing) removed. Height was measured using
a stadiometer with the subject’s shoes removed.
Assessments
The predicted weight and height for each subject and visit were
calculated based on the United States 2000 Centers for Disease
Control (CDC) Growth Charts (ages 2 to <20 years) (Kuczmarski
et al. 2002), using each subject’s observed baseline weight or
height. The predicted weight and height assumed maintenance of
baseline weight and height percentile for each subject. The pre-
dicted weight and height over time were used as references to assess
the trajectory of the observed weight and height over time.
Z-score, which expresses an anthropometric value as the number
of standard deviations (SDs) below or above the mean of a refer-
ence population, was calculated for each subject at each visit. The
reference population was based on the United States 2000 CDC
Growth Charts (ages 2 to <20 years) (Kuczmarski et al. 2002).
Z-scores were assessed against the reference population for both
weight and height (Kuczmarski et al. 2002), as follows:
Z score ¼
observed value mean of the reference population
SD of the reference population
A Z-score change <0.5 SD is considered not clinically signifi-
cant. Z-score change from baseline was calculated based on the
baseline values for the subjects remaining in the study at the ob-
servation time point. Percentiles were assessed as the percentage of
observations that fell below a certain value for the reference popu-
lation. Z-score by baseline body mass index (BMI) quartile was
calculated based on the following baseline BMI quartiles: quartile 1,
BMI £16.1; quartile 2, BMI >16.1 to £17.8; quartile 3, BMI > 17.8
to £21.6; quartile 4, BMI >21.6.
The descriptive statistics included number of subjects (n), mean,
SD, standard error, and Z-score. No statistical tests were conducted.
Results
Subject disposition
Subject disposition has been previously described (Childress
et al. 2023). Briefly, 323 subjects were screened, among whom 282
were enrolled in the study. Of the 282 enrolled subjects, 254 entered
the treatment phase (which included 70 rollover subjects and
184 new subjects), of whom 238 subjects were included in the
treatment phase safety population (Fig. 1).
GROWTH VELOCITY IN CHILDREN TREATED WITH SDX/
D-MPH 135

Subject demographics and baseline characteristics
The mean (SD) age for the subjects was 9.1 (1.9) years; 60.9% of
the subjects were male, and 39.1% were female. The mean (SD;
minimum, maximum) for weight was 38.6 (13.9; 21.0, 97.0) kg,
BMI was 19.2 (4.6; 13.1, 37.5) kg/m
2
, and height was 139.6 (11.9;
113.5, 171.2) cm (Table 1). Additional subject characteristics have
been previously reported (Childress et al. 2023).
Growth velocity
Weight. The mean (SD) body weight at baseline was
38.6 (13.9) kg with a Z-score of 0.74 (1.13) (Table 2), indicating a
baseline weight higher than the mean expected body weight for the
reference population. In the first 30 days after initiating the treat-
ment phase, mean observed weight decreased slightly, and this was
followed by 2 months of slowdown in observed weight gain
(Fig. 2A). After this slowdown, a steady increase in observed body
weight paralleled the predicted weight curve, but at a lower starting
point because of the weight deficits in the study population during
the first 3 months.
At 90 days, the mean observed weight and predicted weight were
38.5 and 40.2 kg, respectively, with a difference between the means
of 1.7 kg. At the end of the study (360 days), the mean observed
weight and predicted weight were 41.1 and 42.8 kg, respectively,
with the same difference between the means of 1.7 kg as after
3 months of treatment.
The mean (SD) weight Z-score at baseline was 0.74 (1.13). Mean
weight Z-score decreased during the first month in the treatment
phase, as evidenced by the decline at 30 days (Fig. 2B). Most of the
mean weight Z-score decline occurred in the first 4 months of
treatment and remained stable thereafter, near 0.5. For all subjects,
the mean (SD) weight Z-score during the first 3 months was
0.53 (1.17), and at 12 months, it was 0.51 (1.08), a change from
baseline of -0.22 (0.31) at 3 months and -0.20 (0.50) at 12 months
based on the baseline scores for those subjects remaining in the
study at 3 and 12 months, respectively.
For subjects who completed the study, smaller decreases or
lower-than-expected increases were observed during the second
and third months, after which the weight velocity resumed, al-
though at a lower starting weight at 90 days. The average weight
decreased from the 68.4 (27.4) percentile to the 64.3 (28.8) per-
centile during the first month in the treatment phase, with an ad-
ditional small decrease to the 62.8 (27.7) percentile by 12 months
(Fig. 2C).
Approximately the same mean weight percentile was maintained
for the remainder of the study. The percentage of subjects with
clinically notable weight loss, defined as a weight decrease from
baseline >7%, was 8.7% at 30 days. The percentage of subjects with
weight loss increased over time, peaking at 14.5% of subjects at
4 months and then declining to 5.2% of subjects at the end of the
study (360 days). This time course is consistent with average
weight loss observed during the beginning of the treatment phase
followed by weight gains later in the study.
The mean weight Z-scores over time by baseline BMI quartiles
are shown in Figure 3. The mean baseline Z-scores were -0.40,
0.32, 0.92, and 2.16, for baseline BMI quartiles 1, 2, 3, and 4,
respectively. At the end of treatment, the mean Z-scores were
-0.46, 0.12, 0.55, and 1.95, a change from baseline of -0.07, -0.13,
-0.33, and -0.25 for baseline BMI quartiles 1, 2, 3, and 4, re-
spectively.
The Z-scores for subjects with a baseline BMI <16.1 kg/m
2
ap-
proached the baseline Z-score by the end of the study after a de-
crease in weight Z-score during the initial 3 months of treatment.
The weight Z-score of subjects with mean BMI > 21.6 kg/m
2
at
baseline decreased during the initial 2 months of treatment and
remained approximately constant thereafter.
Height. The mean (SD) height at baseline was 139.6 (11.9) cm
with a Z-score of 0.54 (0.98) (Table 2), indicating that the baseline
height was higher than the mean expected body height for the
reference population (i.e., the subjects in the present study were
FIG. 1. Subject disposition.
Table 1. Subjects’ Demographics. Treatment Phase
Safety Population
Parameter Total (N = 238)
Age, years
Mean (SD) 9.1 (1.87)
Minimum, maximum 6, 12
Sex, n (%)
Male 145 (60.92)
Female 93 (39.08)
Weight, kg
Mean (SD) 38.6 (13.9)
Minimum, maximum 21.0, 97.0
Height, cm
Mean (SD) 139.6 (11.92)
Minimum, maximum 113.5, 171.2
Body mass index, kg/m
2
Mean (SD) 19.2 (4.6)
Minimum, maximum 13.1, 37.5
SD, standard deviation.
Table 2. Baseline Observed Mean Body Weight
and Height, Predicted Weight and Height, Weight
and Height Z-Score, and Weight and Height
Percentiles: Treatment Phase Safety Population
Parameter, mean (SD)
Weight (kg),
n = 238
Height (cm),
n = 238
Observed 38.6 (13.9) 139.6 (11.9)
Predicted 38.6 (13.9) 139.6 (11.9)
Z-score 0.74 (1.13) 0.54 (0.98)
Percentile 68.4 (27.4) 64.9 (26.6)
SD, standard deviation.
136 CHILDRESS ET AL.

FIG. 2. Mean observed body weight versus mean predicted body weight (A), mean weight Z-score by completion status (B), and mean
body weight percentile (C). Treatment phase, safety population. All subjects: n = 238. Completers: n = 155. The black curve represents
all subjects remaining in the study at each visit (completers + drop-outs before they dropped out). The red curve represents completers
who are subjects that completed 12 months of treatment. Bars are standard error.
137

taller than the expected height for their age, according to CDC
norms).
A comparison of observed height versus predicted height
showed a steady increase of the mean observed height over time,
which was at a lower rate than the predicted velocity of height gain
at each monthly visit (Fig. 4A). In the treatment phase at 90 days,
the mean observed height and predicted height were 140.8 and
141.4 cm, respectively, with a difference between the means of
0.6 cm. At 180 days, the mean observed height and predicted height
were 141.8 and 142.7 cm, respectively, with a difference between
the means of 0.9 cm. At the end of the study, the mean observed
height and predicted height were 143.4 and 144.8 cm, respectively,
with a difference between the means of 1.4 cm.
Mean height Z-scores decreased over time (Fig. 4B). The mean
(SD) baseline height Z-score for all subjects was 0.54 (0.98). The
greatest mean Z-score decrease occurred at 6 months. The mean
height Z-score decreased to 0.39 (0.98) after 6 months in the
treatment phase, a change from baseline of -0.14 (0.28), and de-
creased to 0.24 (0.96) after 12 months, a change from baseline of
-0.21 (0.39), based on the baseline scores for those subjects re-
maining in the study at 6 and 12 months, respectively.
Mirroring the mean height Z-score, the mean height percentile
steadily decreased, with the mean (SD) height percentile decreasing
from the 64.9 (26.6) to the 60.7 (27.7) percentile after 6 months in
the treatment phase and to the 57.0 (27.7) percentile at 12 months
(Fig. 4C).
The mean baseline height Z-scores were 0.18, 0.53, 0.51, and
0.96, for baseline BMI quartiles 1, 2, 3, and 4, respectively (Fig. 5).
At the end of treatment, the mean Z-scores were -0.11, 0.16, 0.19,
and 0.79, a change from baseline of -0.25, -0.25, -0.23, and -0.04,
FIG. 3. Mean weight Z-score by baseline BMI quartile. Treatment phase, safety population. Bars are standard error. BMI, body mass
index.
138 CHILDRESS ET AL.

FIG. 4. Mean observed height versus mean predicted height (A), mean height Z-score by completion status (B), and mean height
percentile (C). Treatment phase, safety population. All subjects: n = 238. Completers: n = 155. The black curve represents all subjects
remaining in the study at each visit (completers + drop-outs before they dropped out). The red curve represents completers who are
subjects that completed 12 months of treatment. Bars are standard error.
139
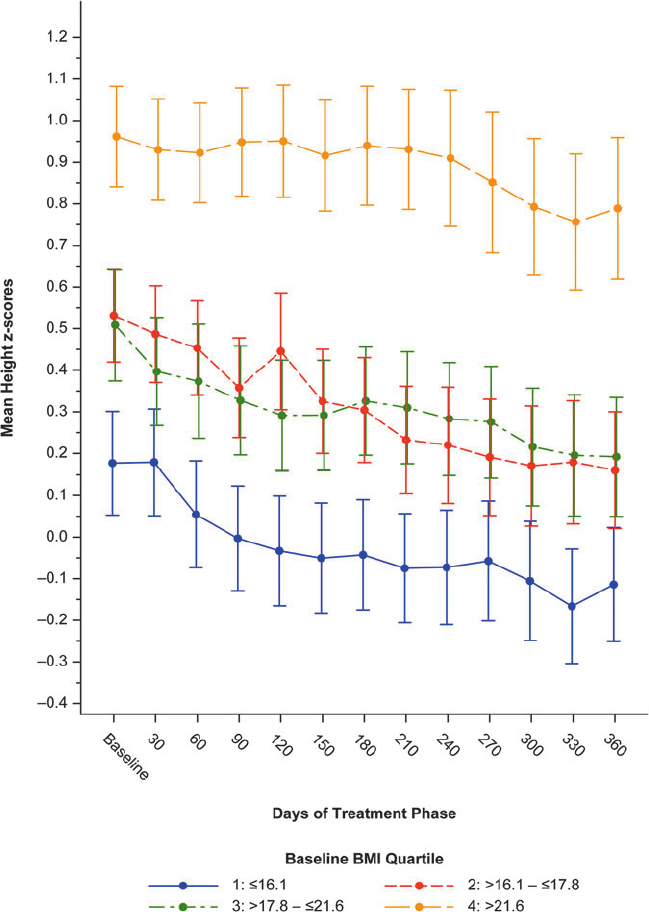
for baseline BMI quartiles 1, 2, 3, and 4, respectively. The mean
height Z-score decrease from baseline was much lower in the
baseline BMI quartile 4 (which also had a higher mean baseline
height Z-score) than in the lower 3 baseline BMI quartiles.
Discussion
The safety and tolerability results from this 1-year open-label
study of SDX/d-MPH in children with ADHD have been previously
reported (Childress et al. 2023). Decreased weight was reported as a
treatment-emergent AE, occurring in 7.6% of subjects. In this
analysis, baseline mean weight and height were higher in study
subjects than in the reference population.
Other studies of MPH in children with ADHD also observed
similarly higher baseline weight and height than their reference
samples (Swanson et al. 2006; Childress et al. 2022). In this anal-
ysis, we showed a transient decrease in observed body weight
followed by a slower-than-predicted weight gain in the first
3 months of SDX/d-MPH treatment. Subsequently, the resulting
difference between observed and predicted mean body weights
remained fairly constant to the end of the treatment phase. In the
present study, the change in weight Z-score from baseline to the
study’s end at 12 months was -0.20.
A Z-score change <0.5 SD is considered not clinically signifi-
cant. The mean weight Z-score decreased initially, with the largest
mean Z-score decrease occurring during the first month of the
treatment phase, with smaller decreases in the second and third
month. These results are consistent with published data, suggesting
that the rate of accumulating weight deficits is attenuated over time
with continued stimulant treatment (Faraone et al. 2008).
FIG. 5. Mean height Z-score by baseline BMI quartile. Treatment phase, safety population. Bars are standard error. BMI, body mass
index.
140 CHILDRESS ET AL.
The mean weight changes as a percent from baseline-by-
baseline BMI quartile groups showed that all four baseline BMI
quartile groups had an initial weight decrease during the first to
fourth months in the treatment phase, with a notable smaller and
shorter weight decrease in baseline BMI quartile 1 compared with
the other three baseline BMI quartile groups.
The relative weight gain after the initial decline through the end
of treatment was higher in subjects in the first and second baseline
BMI quartile groups compared with subjects in the third and fourth
baseline BMI quartile groups. This difference in weight gain in-
dicates that subjects with a higher baseline BMI experienced a
larger initial reduction in weight gain than subjects with a lower
baseline BMI. Relative weight gain after *3 months of treatment
appeared similar between all baseline BMI quartiles.
With respect to height, the positive mean baseline height Z-score
of 0.54 for all study subjects indicates that the average baseline
height was higher than the mean expected height for the reference
population. Mean height increased steadily during the study, al-
though the height increases were lower when compared against the
reference population. Mean height Z-scores and height percentiles
decreased over time.
The change in height Z-score from baseline to the end of the
study was -0.21. We also showed that the mean height Z-score
decrease from baseline was much lower in the upper baseline BMI
quartile group (-0.04 for fourth baseline BMI quartile) than in the
lower three baseline BMI quartiles (-0.25, -0.25, and -0.23 for
first, second, and third baseline BMI quartile groups, respectively).
The effects of long-term treatment of stimulants on weight and
height in pediatric patients are well documented (Spencer et al.
2006; Swanson et al. 2006; Faraone et al. 2008, 2010). A 5-week
study of 253 children aged 6–12 years with ADHD receiving daily
doses of extended release d-MPH found that the percentage of
subjects with clinically notable weight loss (a weight decrease from
baseline >7%) was 5.8%, 4.0%, and 7.7%, after daily doses of 10,
20, and 30 mg extended release d-MPH (vs. 0% after placebo)
(Childress et al. 2009).
However, that study was of a much shorter span than the current
study, consisting of a 3-week dose optimization phase followed by
a 2-week maintenance phase, and it is possible that study had not
reached maximum effect on body weight that may have been
measured later in treatment had the treatment continued.
In a 21-month study with a dose-optimized long-acting MPH
formulation in 407 children aged 6–13 years with ADHD (Spencer
et al. 2006), the mean baseline weight Z-score was higher than 0,
indicating that the children in that study were on average heavier
than the CDC reference sample. In that study, the largest weight
Z-score decrease occurred during the first 5 months of treatment
(decreased from 0.16 to -0.11) and then remained relatively stable
for the remainder of the study.
In a more recent 12-month open-label safety study of extended-
release MPH in children aged 4–5 years with ADHD, subjects
entering the study were heavier and taller than the reference pop-
ulation (Childress et al. 2022). Weight decrease was a commonly
reported treatment-emergent AE (Childress et al. 2022), as was
found in the 1-year safety study of SDX/d-MPH (Childress et al.
2023).
In the extended-release MPH study (Childress et al. 2022), de-
crease in weight-for-age percentiles was smallest for subjects in the
lowest quartile of baseline weight (-8.1) versus the other three
baseline weight quartiles (-14.0, -10.0, and -11.4 for second, third,
and fourth quartiles, respectively). In addition, the smallest changes
in height percentiles were seen for children in the first quartile of
baseline height (-2.4), compared with the second (-10.0), third
(-8.7), and fourth (-9.7) baseline height quartiles.
The limitations of this study include the open-label nature of the
study design and the lack of a control placebo arm or a comparator
product. The analysis of a drug effect on body weight changes over
time by itself is limited because children are expected to grow and
gain weight as they age, even during SDX/d-MPH treatment. In
addition, some of the subjects who entered this study were not
stimulant naive, as some had been previously treated with SDX/d-
MPH in the antecedent pivotal study. Thus, previous stimulant
exposure may have impacted baseline height and weight measures,
resulting in an underestimate of growth velocity changes.
Conclusion
In this study, we show that long-term treatment for up to 1 year
with SDX/d-MPH was associated with modest reductions in ex-
pected weight and lower-than-expected increases in height, effects
that plateaued or diminished later in treatment. Although both
weight and height decreased compared with the reference popula-
tion, the decrease was less than what is clinically significant.
Clinical Significance
This phase 3 clinical trial of SDX/d-MPH in children aged
6–12 years showed that the overall effects of SDX/d-MPH on
growth velocity were minimal and changes were in a range con-
sidered not clinically significant.
Acknowledgments
Medical writing support for the development of this manuscript,
under the direction of the authors, was provided by Gautam Bijur,
PhD, Charlette Tiloke, PhD, and editing support by Kathleen
Blake, PhD, all of Ashfield MedComms, an Inizio company, and
funded by Corium, LLC.
Authors’ Contributions
All the authors were actively involved in data interpretation,
critical review of the data, article writing, and editing.
Disclosures
A.C.C serves as consultant for Aadrvark, Arbor, Aytu, Iron-
shore, Neos Therapeutics, Neurocentria, Noven, Otsuka, Purdue,
Rhodes, Sky, Sunovion, Tris Pharma, Zevra Therapeutics (previ-
ously KemPharm, Inc.), Supernus, Jazz, Corium, LLC, and Lumos.
She has served on the speaker’s bureau for Takeda (Shire), Arbor,
Ironshore, Neos Therapeutics, Tris Pharma, and Supernus. She has
received research support from Allergan, Takeda (Shire), Emalex,
Akili, Arbor, Ironshore, Lumos, Neos Therapeutics, Otsuka, Pur-
due, Adlon, Rhodes, Sunovion, Tris Pharma, Zevra Therapeutics
(previously KemPharm, Inc.), Supernus, US Food and Drug Ad-
ministration, and Servier. She has received writing support from
Takeda (Shire), Arbor, Ironshore, Neos Therapeutics, Purdue,
Rhodes, Sunovion, and Tris Pharma; She has served on advisory
boards for Takeda (Shire), Akili, Arbor, Cingulate, Ironshore, Neos
Therapeutics, Neurovance, Otsuka, Purdue, Adlon, Rhodes, Su-
novion, Tris Pharma, Supernus, NLS Pharma, and Corium, LLC.
A.J.C. is a consultant for AbbVie, Akili Interactive, Arbor
Pharmaceuticals, Atentiv, Axsome, Corium, LLC, Ironshore,
Neurosigma, Noven, Otsuka, Purdue Canada, Shire, Sunovion,
Supernus, Takeda, and Tris Pharma. He has served on speaker’s
GROWTH VELOCITY IN CHILDREN TREATED WITH SDX/
D-MPH 141
bureaus for AbbVie, Arbor Pharmaceuticals, Axsome, Corium, LLC,
Ironshore, Noven, Otsuka, Shire, Sunovion, Supernus, Takeda, and
Tris Pharma. He has received research support from Aevi Genomics,
Akili Interactive, Arbor Pharmaceuticals, Ironshore, Zevra Ther-
apeutics (previously KemPharm, Inc.), Otsuka, Purdue Canada,
Rhodes, Shire, Sunovion, Supernus, and Takeda. He is an employee
and board member of the Neuroscience Education Institute.
M.P. and C.O. are employees of Corium, LLC.
References
Childress AC, Foehl HC, Newcorn JH, Faraone SV, Levinson B,
Adjei AL: Long-term treatment with extended-release methylphe-
nidate treatment in children aged 4 to <6 years. J Am Acad Child
Adolesc Psychiatry 61:80–92, 2022.
Childress AC, Marraffino A, Cutler AJ, Oh C, Brams MN: Safety and
tolerability of serdexmethylphenidate/dexmethylphenidate capsules
in children with attention-deficit/hyperactivity disorder: A 12-
month, open-label safety study. J Child Adolesc Psychopharmacol
33:51–58, 2023.
Childress AC, Spencer T, Lopez F, Gerstner O, Thulasiraman A,
Muniz R, Post A: Efficacy and safety of dexmethylphenidate
extended-release capsules administered once daily to children with
attention-deficit/hyperactivity disorder. J Child Adolesc Psycho-
pharmacol 19:351–361, 2009.
Faraone SV, Biederman J, Morley, Spencer TJ: Effect of stimulants
on height and weight: A review of the literature. J Am Acad Child
Adolesc Psychiatry 47:994–1009, 2008.
Faraone SV, Giefer EE: Long-term effects of methylphenidate
transdermal delivery system treatment of ADHD on growth. J Am
Acad Child Adolesc Psychiatry 46:1138–1147, 2007.
Faraone SV, Spencer TJ, Kollins SH, Glatt SJ: Effects of lisdex-
amfetamine dimesylate treatment for ADHD on growth. J Am Acad
Child Adolesc Psychiatry 49:24–32, 2010.
Kollins SH, Braeckman R, Guenther S, Barrett AC, Mickle TC, Oh C,
Marraffino A, Cutler AJ, Brams MN: A randomized, controlled
laboratory classroom study of serdexmethylphenidate and
d-methylphenidate capsules in children with attention-deficit/
hyperactivity disorder. J Child Adolesc Psychopharmacol 31:597–
609, 2021.
Krinzinger H, Hall CL, Groom MJ, Ansari MT, Banaschewski T,
Buitelaar JK, Carucci S, Coghill D, Danckaerts M, Dittmann RW,
Falissard B, Garas P, Inglis SK, Kovshoff H, Kochhar P, McCarthy
S, Nagy P, Neubert A, Roberts S, Sayal K, Sonuga-Barke E, Wong
ICK, Xia J, Zuddas A, Hollis C, Konrad K, Liddle EB: Neurolo-
gical and psychiatric adverse effects of long-term methylphenidate
treatment in ADHD: A map of the current evidence. Neurosci
Biobehav Rev 107:945–968, 2019.
Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal
KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL: 2000 CDC
growth charts for the United States: Methods and development.
Vital Health Stat 11:1–190, 2002.
Spencer TJ, Faraone SV, Biederman J, Lerner M, Cooper KM, Zim-
merman B: Does prolonged therapy with a long-acting stimulant
suppress growth in children with ADHD? J Am Acad Child Ado-
lesc Psychiatry 45:527–537, 2006.
Storebø OJ, Pedersen N, Ramstad E, Kielsholm ML, Nielsen SS,
Krogh HB, Moreira-Maia CR, Magnusson FL, Holmskov M, Ger-
ner T, Skoog M, Rosendal S, Groth C, Gillies D, Rasmussen KB,
Gauci D, Zwi M, Kirubakaran R, Ha
˚
kosen SJ, Aagaard L, Si-
monsen E, Gluiud C: Methylphenidate for attention deficit hyper-
activity disorder (ADHD) in children and adolescents—Assessment
of adverse events in non-randomised studies. Cochrane Database
Syst Rev 5:CD012069, 2018.
Swanson J, Greenhill L, Wigal T, Kollins S, Stehli A, Davies M,
Chuang S, Vitiello B, Skrobala A, Posner K, Abikoff H, Oatis M,
McCracken J, McGough J, Riddle M, Ghuman J, Cunningham C,
Wigal S: Stimulant-related reductions of growth rates in the PATS.
J Am Acad Child Adolesc Psychiatry 45:1304–1313, 2006.
Address correspondence to:
Charles Oh, MD
Corium, LLC
11 Farnsworth St., FL 4
Boston, MA 02210
USA
E-mail: [email protected]
142 CHILDRESS ET AL.
