
저작자표시-비영리-변경금지 2.0 대한민국
이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게
l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다.
다음과 같은 조건을 따라야 합니다:
l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건
을 명확하게 나타내어야 합니다.
l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다.
저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다.
이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다.
Disclaimer
저작자표시. 귀하는 원저작자를 표시하여야 합니다.
비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다.
변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.

2023년 08월
석사학위논문
보스웰리아 (Boswellia serrata)
추출물의 향장소재 적용을
위한 항산화와 항균활성
조 선 대 학 교 산 업 기 술 창 업 대 학 원
미 용 향 장 학 과
바 스 마

보스웰리아 (Boswellia serrata)
추출물의 향장소재 적용을
위한 항산화와 항균활성
Antioxidant and Antimicrobial Activity of
Boswellia Serrata Extract for Cosmetic
Ingredient
2 0 2 3 년 0 8 월 2 5 일
조 선 대 학 교 산 업 기 술 창 업 대 학 원
미 용 향 장 학 과
바 스 마

보스웰리아 (Boswellia serrata)
추출물의 향장소재 적용을
위한 항산화와 항균활성
지 도 교 수 신 현 재
이 논문을 미용향장학 석사학위신청 논문으로 제출함
2 0 2 3 년 0 4 월
조 선 대 학 교 산 업 기 술 창 업 대 학 원
미 용 향 장 학 과
바 스 마


I
Contents
List of Tables ··································································· Ⅳ
List of Figures ·································································· Ⅴ
ABSTRACT ······································································· Ⅵ
Ⅰ. Introduction ··································································· 1
1.1. Background of the study ······················································ 1
1.1.1. Herbal cosmetics ····································································· 1
1.1.2.
Boswellia Serrata
······························································ 3
1.1.3. Scientific classification ··························································· 5
1.1.4. Economic importance ··························································· 12
1.2. Antioxidant ················································································· 18
1.2.1. Definition..................................................................................18
1.2.2. Free Radicals and Oxidative Stress ...................................19
1.2.3. Skin antioxidant ....................................................................19
1.3. Antimicrobial.................................................................................22
1.3.1. Defense systems of the skin .................................................22

II
1.3.2. Bacterial growth on skin .....................................................23
1.3.3. Protection by colonizing bacteria..........................................23
1.3.4. Microbes that cause skin disease ........................................24
1.3.5. Antibacterial .............................................................................27
1.4. Research Trends and Composition ···································· 28
1.4.1. Research Trends ···································································· 28
1.4.2. Structure of the study ·························································· 30
Ⅱ. Literally review ·························································· 31
2.1. Review of research on
Boswellia serrata
····················· 31
2.1.1. Ingredients and compounds of
Boswellia serrata
······· 31
2.1.2. Bioactive compounds in
Boswellia serrata
················· 36
2.1.3.
Boswellia serrata
in cosmetology ································ 39
2.2. A review of research on
Boswellia serrata
················· 47
Ⅲ. Materials and Methods ·············································· 50
3.1. Experimental Materials and Reagents ······························· 50
3.1.1. Materials ········································································ 50
3.1.2. Reagents ········································································ 50
3.2. Extraction and Isolation ····················································· 51

III
3.2.1. Sample collection ·································································· 51
3.2.2. Extraction ··············································································· 51
3.2.2.1 Soxhlet Extraction ······························································ 51
3.2.2.2 Immersion Extraction ························································· 53
3.2.3. floor separation ····································································· 55
3.3. Antibacterial activity ··························································· 56
3.3.1. Antibacterial agar disc diffusion assay ······························ 56
3.4. Antioxidant activity ......................................................................57
3.4.1. DPPH free radical scavenging assay..................................57
3.4.2. ABTS radical scavenging assay............................................59
3.5. Total polyphenol and flavonoid contents...............................61
3.5.1. Determination of total polyphenol content....................61
3.5.2. Determination of total flavonoid content.........................62
3.6. Analysis of polyphenol componds...................................................63
3.6.1 High performance liquid chromatography (HPLC) analysis63
3.6.2 HPLC-MS/MS Analysis Method.............................................64
Ⅳ. Results and Discussion ············································· 65
4.1. Yield of extract according to Immersion extraction method.......65
4.2. Antibacterial activity using well diffusion method ··················· 68

IV
4.3. Results of antioxidant activity................................................71
4.3.1. DPPH free radical scavenging............................................71
4.3.2. ABTS radical scavenging....................................................73
4.4. Results of total polyphenol and flavonoid contents............75
4.4.1 Total polyphenol contents (TPC).........................................75
4.4.2. Total flavonoid contents (TFC)...........................................76
4.5. Analysis results of polyphenol componds.....................................78
4.5.1 HPLC Analysis Results........................................................78
4.5.2 HPLC-MS/MS Analysis Results...........................................81
Ⅴ. Discussion.....................................................................86
Ⅵ. Conclusion ································································ 89
Ⅶ. Reference ·································································· 90

V
List of Tables
Table 1. Taxonomical Hierarchy of
Boswellia serrata
·················································· 7
Table 2. Vernacular names of
Boswellia serrata
··························································· 8
Table 3. products containing B. Serrata resin extracts in cosmetics..............................15
Table 4. The most common microbial infection...............................................................25
Table 5. Main ingredients and compounds in
Boswellia serrata
··························· 38
Table 6. Chemical composition of
Boswellia serrata
. resin and use as cosmetic
ingredients.............................................................................................................43
Table 7. The yield of each Fractions according to the extracted Boswellia Serrata...67
Table 8. Antibacterial activity of B. Serrata using the disk diffusion method ········· 69
Table 9. Radical scavenging activity and polyphenolic/Flavonoids content of different
Boswellia fractions................................................................................................77
Table 10. Polyphenol compounds identified in Boswellia serrata extracs quantified by
HPLC (Unit :μg/mg).............................................................................................79
Table 11. Polyphenol compounds identified in Boswellia serrata extract quantified by
HPLC-MS/MS........................................................................................................82

VI
List of Figures
Figure 1. Tree parts of
Boswellia Serrata
................................................................9
Figure 2.
Boswellia Serrata
resin extracted method......................................................10
Figure 3. The four grades of
Boswellia Serrata
............................................................11
Figure 4. products containing
Boswellia Serrata
.resin extracts...................................17
Figure 5. Environmental causes causing the formation of free radicals.......................21
Figure 6. Chemical structures of boswellic acids............................................................35
Figure 7. The illustrated list of cosmetic applications of
Boswellia serrata
..........42
Figure 8. Soxhlet Extraction diagram of
Boswellia Serrata
....................................52
Figure 9. Immersion Extraction diagram of Boswellia serrata ······································ 54
Figure 10. Measurement of the activity of an antioxidant by the DPPH assay...........58
Figure 11. Measurement of the activity of an antioxidant by the ABTS assay...........60
Figure 12. Floor separation diagram of Boswellia Serrata..............................................66
Figure 13. Antibacterial activity of Boswellia Serrata of different concentrations
against M.pachydermatis, M.furfur, S.epidermidis, and C.acnes using the
disk diffusion method.......................................................................................70

VII
Figure 14. DPPH free radical scavenging activity results of Boswellia Serrata
extract.................................................................................................................72
Figure 15. ABTS radical scavenging activity results of Boswellia Serrata
extract.................................................................................................................74
Figure 16. HPLC profile of Boswellia serrata extracts and standard mixture using
diode array detection at 280 nm. (A) Boswellia serrata water extract; (B)
Boswellia serrata 70% EtOH extract; (C) Boswellia serrata EtOAc
extract; (D) standard mixture. Numbers indicate the following: (1) gallic
acid; (2) catechin; (3) (-)-epicatechin; (4) vanillic acid; (5)Narigin;
(6)Ethyl gallate; (7) p-coumaric; (8) ferulic acid; (9) benzoic acid; (10)
quercetin; (11) narigenin; (12) kaempferol; (13) 4-hydroxybenzoic
acid.....................................................................................................................80
Figure 17. Component analysis of Boswellia serrata water extract by HPLC
MS/MS...............................................................................................................83
Figure 18. Component analysis of Boswellia serrata 70% EtOH extract by HPLC
MS/MS...............................................................................................................84
Figure 19. Component analysis of Boswellia serrata EtOAc extract by HPLC
MS/MS...............................................................................................................85

VIII
ABSTRACT
Antioxidant and Antimicrobial Activity of Boswellia Serrata
Extract of Cosmetic Ingredient
Bssmah Ghazi Alraddadi
Advisor: Prof. Hyun-Jae Shin, Ph.D.
Department of Beauty and Cosmetology,
Graduate School of Industrial Technology and Entrepreneurship,
Chosun University
Boswellia serrata resin, which is an important source of gum oleoresin known as
Indian frankincense and is well documented for its pharmaceutical properties due to its
chemical structure, antibacterial and antioxidant properties, and the presence of several
compounds such as polyphenols, phenols, and terpenoids.
In the experimental study, it was observed that Boswellia Serrata resin extracts in-
hibited antibacterial activity in all strains for the relevant concentrations. The diameter
of the zone of inhibition for the B. serrata extract for S. epidermidis ranged from
13.3±0.58 to 10.3±0.58 mm, for M. furfur from10.6±0.58 to 9±0 mm, for M. pachy-
dermatis from13.25±0.35 to 9.75±1.06 mm, and for C. acnes from 11.83±0.29 to 9.5±0
mm in the anaerobic jar.
antioxidant activity was measured with DPPH It was confirmed that there was high-
er activity in the Water Fr. compared to the Other fractions; respectively, Water Fr.

IX
showed the highest scavenging activity 902.19 ± 35.53 µg/mL, Ethyl acetate Fr. 20436
± 652.19 µg/mL ,70% EtOH Fr. 8627.74± 369.22 µg/mL, and the Hexane Fr. Shows
No scavenging activity.
According to ABTS assay, the Water fraction demonstrated the maximum scaveng-
ing activity with a scavenging activity of 1845.08 ± 2265.74, followed by the ethyl
acetate fraction with a scavenging activity of 12167.16 ± 8152.82 µg/mL.
polyphenol and flavonoid content in the Water Fr. was the highest; it reached TPC
32.15 ± 0.75 ⅿg/mL and TFC 20.29 ± 1.47 ⅿg/mL.
This study shows that the Boswellia serrata resin have some biological activities, and
if they differ in terms of effectiveness and activity, they can be considered a good
component of cosmetic products.

1
Ⅰ. Introduction
1.1. Background of the study
1.1.1. Herbal cosmetics
Herbal cosmetics are products manufactured with phytochemicals from various bota-
nical sources that affect skin functions and provide nutrients for healthy skin or hair[1]
Cosmetics are materials that are intended to be rubbed, poured, sprinkled, sprayed, in-
jected into, or otherwise applied to the human body or any component of it for wash-
ing, beautifying, promoting attractiveness, or changing the appearance[2] Herbal cos-
metics are products made with one or more herbal substances legally used to provide
specific cosmetic benefits exclusively and a basis for various cosmetics[3] Active com-
pounds found in medicinal plants are substances produced by the plant's natural metabo-
lism and are crucial in treating many human illnesses, particularly infections brought on
by bacteria[4] Plant antimicrobials contribute significantly to the eradication of infections
caused by pathogenic microbes[5] These active components have multiple functions, in-
cluding improving skin elasticity, preventing collagen degradation, protecting against UV
radiation by antioxidant property and delaying the aging process of the skin by smooth-
ing out wrinkles[6] Individuals' skin and hair beauty are influenced by their health, rou-
tines, daily activities, environment, and upkeep[7] Utilizing a range of herbs and plants,
the science of Ayurveda enabled the development of efficient Ayurvedic cosmetics.
Ayurvedic cosmetics adorn the face and shield the body from external impacts[8] Many
herbal cosmetics are developed and extensively used in daily life. Herbal cosmetics like
facial washes, conditioners, soaps, shampoos, and others are popular with the general
public. Their best quality is that only herbs and shrubs make herbal cosmetics. Plant
cosmetics, often known as ayurvedic, still have the same beneficial properties.
Additionally, instead of harming the body, the natural elements of the herbs nourish it
with nutrients and other advantageous minerals[8] Since the beginning of the practice of
medicine, natural materials with such a plant origin have been used in healthcare. The
evaluation of phytochemicals for pharmaceutical development has been widespread in re-
cent decades. However, only a tiny handful of these plant species have received a thor-

2
ough scientific inspection. Therefore, research into the bioactivities of these plants and
compounds is necessary. Even today, some of these historically used plants and prod-
ucts derived from plants are still important pharmacologically. One such healing herb is
Boswellia serrata
(Burseraceae)[9]

3
1.1.2.
Boswellia serrata
Natural resins have played an essential role since ancient times, as they were consid-
ered among the plants with primary resources for food, flavors, and aromas. They are
considered high-value ingredients for being an important component in the manufacture
of human medicines. These adhesives have also been utilized as coating materials, cos-
metic compounds, fragrances in religious ceremonies and daily rituals, and for their dif-
ferent medicinal properties[10]
Boswellia serrata
resin were used extensively in ancient times by the Hindus,
Babylonians, Persians, Romans, Chinese, Greeks and early Americans for incense and
embalming. They firmly believed that the smoke and aroma produced by these materials
when burned with fire not only helped to lift their spirits but also to appease their
gods. Their collective life revolves largely around the burning of these natural resins.
To prevent evil spirits from affecting their spirits or to commemorate the dead or the
living, they burned these resins during sacrifice ceremonies and as part of their daily
rituals[11] Since ancient times, these species have been utilized for their medicinal and
aromatic qualities. As a result, the ethnobotany of their immediate surroundings fre-
quently emphasizes the importance of their trees and shrubs. The resins of this species
have been used by indigenous communities for various purposes in traditional medicine,
including as an antiseptic and disinfectant, an external agent (cosmetics), and a wound
dressing[12]
Frankincense is a significant oleo-gum resin used in various industries, including the
pharmaceutical, culinary, perfumery, flavoring, liqueur, beverages, cosmetics businesses.
Since ancient times, people have valued frankincense for its ceremonial and sacred pur-
poses, even before Biblical times[13] is one of the most popularly used essential oils in
aromatherapy to treat breathing disorders. It facilitates respiration and benefits people
with asthma. Additionally, it relieves the symptoms of colds, asthma, bronchitis, and
laryngitis[14] used to treat various bacterial and fungal infections[15] utilized to cure
malignant disorders as well based on numerous studies have demonstrated its efficiency

4
in combating human leukemia[16]
Due to its ability to regulate the release of immune cytokines and the presence of
cortisone, which inhibits inflammation and does not have the adverse side effects asso-
ciated with synthetic cortisone, frankincense resin is used in the treatment of a variety
of illnesses as well as to strengthen the heart and brain, and treats forgetfulness, blood
diarrhea, treat arthritis, and other infections[17]
For its fresh, balsamic, dry, resinous, slightly green note, frankincense oil is em-
ployed in perfumery as a fixative and in oriental bases, ambers, flowers, colognes, and
manly scents[14] Due to its sweet scent, it is also used as incense[18]

5
1.1.3. Scientific classification
The family Burseraceae has between 560-600 species spread across 18 genera. This
family (Burseraceae) is where Boswellia serrata resin, also known as olibanum or
frankincense, The genus Boswellia is a little one with roughly 28 species [19] In honor
of Johann Boswell, who identified 25 different Boswellia species, the genus Boswellia
was named after his name[20] The Taxonomical Hierarchy and vernacular names of
Boswellia serrata are given in table1, table2
Humans have used botanical remedies like the Indian frankincense tree (Boswellia
serrata) as remedies since ancient times. It has significantly influenced the treatment of
several diseases. A medium-sized deciduous tree known as Boswellia serrata is mainly
found in Saudi Arabia, Oman, Southern Arabia, Yemen, India, Pakistan, Africa, Nigeria,
Somalia, and Asia.
A medium to a big tree, the
Boswellia serrata
can reach heights of 18 meters and
a width of 2.4 meters. The leaves have opposing leaflets and are imparipinnate and
packed together at the ends of the stems, and are frequently serrated. The tiny, white
flowers on this hermaphrodite plant develop in racemes at the axils. Its thin, green-
ish-gray bark turns yellow or crimson as the plant's petals ripen that are imbricated,
number from three to five, and ultimately take on the color of ash. The bark will
exude an exudate containing oleo gum resin after being hurt or having a natural crack.
The bark secretes tiny droplets of resin and sheds in brilliant crimson, papery, silky
flakes. Golden yellow, clear, aromatic oleo gum resin ultimately turns into crusts, tears,
or drips that are brownish-yellow [19] (Figure1) Frankincense resin is harvested from
the tree by creating an incision or wound in its bark that looks like milk or resin, then
dries outside to appear like olibanum[23] (Figure2) which is then kept in a bamboo
basket constructed especially for storage. The semi-solid gum resin is left in the basket
for about a month while the fluid inside flows out. The gum resin, the semi-solid to
the solid portion of the residue, progressively solidifies to form amorphous, tear-shaped
products with an aromatic scent. It is then manually cleaned of all impurities, such as
bark fragments, during the process of being broken into tiny bits with a wooden mallet
or chopper. After that, the gum-resin is rated based on its flavor, color, form, and

6
size[22] Color, scent, cluster size, tree age, and purity, harvest season, and geographic
position of the plant source are some of the variables that affect the quality and type
of luban (Frankincense resin) is offered commercially in four grades, under the Arabic
names Hoojri, Najdi, Shathari, and Shaabi (Figure3) There are four geographical loca-
tions in Oman, specifically the Dhofar region, where the resins is harvested. Hoojri
Grade I resins, is distinguished by its lighter color and larger mass size than the rest
of the species. This type it is gathered from trees that grow in the north of the
Samhan Mountains. It costs $83 per kilogram "It is the sample used in the present
work". While the Grade II, which is Najdi, is distinguished by its pale yellow color. It
is gathered from a plateau that is hidden by mountains of Dhofar. It costs 67$ per kg.
Shathari, Grade III resin it is darker color collected from northwest Dhofar, costs $31
per kilogram. Finally, Shaabi, Grade IV , which is also darker color and collected
from valleys, costs only $15 per kilogram[14]

7
Kingdom Plantae-Plants
Division Angiospermae
Class Dicotyledoneae
Order Geraniales
Family Burseraceae
Genus Boswellia
Species Serrata
Table 1. Taxonomical Hierarchy of Boswellia serrata [21]

8
English Indian olibanum or Indian frankincense
Arabic Luban or Luban dhakar or Luban mur or luban omani
Hindi Kundur, salai
Bangali Kundur, salai
Gujarati Dhup, Gugali
Kannada Chitta, Guguladhuph
Malayalam Parangi, Saambraani
Tamil Parangi, Saambraani
Telugu Phirangi, Saambraani
Sanskrit Ashvamutri, Kundara, Shallaki
Table 2. Vernacular names of Boswellia serrata [22]

9
Figure 1. Tree parts of
Boswellia Serrata
.

10
Figure 2.
Boswellia Serrata
resin extracted method.

11
Figure 3. The four grades of
Boswellia Serrata
.

12
1.1.4. Economic importance
Arabs, Middle Easterners, Indians and even Africans are interested in the Boswellia
serrata plant, because of its social, religious and economic importance in their
countries.
It is used on a daily basis, especially in the homes of Arabs, because they believe
that it helps to clean the house of bad energy and toxins. However, it helps to im-
prove mood and get rid of anxiety.
Also, based on its social standing, it is given as a very precious gift on special
occasions.
Oman is the country that produces the most Boswellia serrata plant, and frankincense
is called Omani because of the interest of the Arabs in frankincense.
There is a special tourism in the Dhofar region in Oman to buy the finest types of
Omani frankincense, which they call (incense and perfume tourism).
Subsequently, the world's attention became focused on Boswellia serrata, as
many pharmaceutical, cosmetic, and fragrance industries include the Boswellia serrata
plant.
a. medical uses
The resin is one of the most important herb medicines with remarkable efficacy in
many therapeutic fields[24] Asthma, Crohn's disease, osteoarthritis, rheumatoid arthritis
are the most prominent medical conditions that B. Serrata is used to treat. In addition,
it's employed to cure bronchitis and cough[19] Use for skin conditions, corneal ulcers,
osteoarthritis, dysentery, inflammatory conditions that last a long time, wound recovery,
and diarrhea[25] used as a weight-loss aid[26] and is advantageous for liver fibrosis[27]
and several cancers[19]Colitis and ulcerative colitis are both treated and prevented with
Boswellia resin. In the cerebrovascular system, B. Serrata exhibits adequate antioxidant

13
activity[28] used for antipyretic, anti-sclerotic, and analgesic[29] in pain, arthritis, in-
cluding osteoarthritis, allergies, and inflammatory bowel illness[30] It has additionally
been utilized in mouthwashes as an antimicrobial[31] also for the treatment of chronic
inflammatory illnesses[32] or the avoidance of skin and nail diseases[33] also benefits
of antiulcer, antiulcerogenic, antibacterial, and psychopharmacological effects[34]
b. Industrial uses
Cosmetology:
Cosmetic industries have become one of the leading industries in developing countries, as
these industries contribute to the country's economic growth.
International cosmetic companies depend on manufacturing skin, hair, and body care prod-
ucts from natural plant sources. On healthy skin, a look that is free from skin infections
that affect most people.
There are numerous applications for Boswellia serrata resin in cosmetics, including
anti-aging, soothing, and anti-inflammatory properties. It can also encourage quicker skin
regeneration and increase skin elasticity to lighten the skin. Some of the global cosmetic
products includes B. Serrata resin extracts are compiled in a table 3.
Perfumery:
Frankincense is of very high value in the manufacture of scented products due to its
beautiful aromatic scent, as it is an essential ingredient in the manufacture of incense,
perfumes, potpourri, creams, lotions, soaps, and detergents. It is frequently incorporated
into meditation combinations since it bolsters the soul and aids in achieving a deeper
level of meditation and relaxation[35] Because it holds its aroma for so long—some
say indefinitely—frankincense is a favorite ingredient in potpourris. These days, it is
still used for making incense, perfume, and medicines. Additionally, it is a component
of numerous contemporary perfumes[36] It is known to be helpful as a fixative in pot-

14
pourris and perfumes in addition to providing a unique fragrance to any blend. It is
utilized by perfumers in Oriental bases, ambers, powder perfumes, floral perfumes, cit-
rus colognes, spice blends, violet perfumes, manly fragrances, soaps, lotions, and
creams, among other things, as an absolute (by alcohol extraction), oil, or resinoid (by
hydrocarbon extraction)[35]
Food and beverages:
A few businesses that employ frankincense products include the beverages, con-
fectioneries, gelatins, nut goods, puddings, cans of vegetables, candies, chewing gum,
and most of food industry. In addition, they are frequently utilized in rubber products
as releasing agents, adhesive thickeners, stabilizers, flavor enhancers, fixing and emulsi-
fying substances in culinary products. 500 tonnes or so of olibanum are imported into
the Middle East, especially Saudi Arabia, to produce chewing gum[35]

15
Product Name Function
united states of America
Aveda—outer peace™ acne relief pads
Eliminate blackheads and stop fresh
breakouts from occurring.
I image—CLEAR CELL clarifying sal-
icylic tonic
Assist in calming sensitive skin and
refresh and clear congested pores.
Aveda—men pure-formance™ condi-
tioner
Refreshes scalp.
Dermadoctor—Ain’T Misbehavin’
Intensive 10% Sulfur Acne Mask &
Emergency Spot Treatment
Reduces the appearance of blackheads
and spots. Additionally, it aids in skin
healing.
Flexpower—Soothe Lotion Soothes, anti-inflammatory.
Found—Marshmallow Calming Face
Serum
Skin conditioning.
Aveda—Outer Peace™ Foaming
Cleanser
Thorough pore cleaning without caus-
ing skin irritation or excessive drying.
Kate Ryan—Collagen Booster Intense
Repair Serum
Reduces lines and wrinkles.
LANCER—Soothe and Hydrate Serum
Balances skin tone while reducing skin
redness.
Skin Actives—Collagen Serum
Enhances the texture and tone of the
skin.
Kate Somerville—Liquid Exfolikate Soothes skin.
New Vitality Lumatone—Anti-Aging
Eye Cream
Antiaging.
Table 3. products containing B. Serrata resin extracts in cosmetics

16
Product Name Function
Asia countries (South Korea, Japan, Indonesia, Malaysia, Thailand)
Smooth-E—Acne Treatment Hydrogel
Reduce acne inflammation, treat
acne, and kindly calm the skin.
Dermedics—YOUTH EXPERT™ Instant
Relief Eye Serum
Anti-irritant.
Dermedics—MESO CALM Instant
Soothing Elixir
Relieves irritability.
Dermedics—YOUTH EXPERT™
Physiological Micellar Water
Anti-irritant.
NPURE—Day Cream Centella Antiacne.
Mitomo—Hyaluronic Acid +
Lithospermum Facial Essence Mask
Brightening, moisturizing, and
refining.
Yadah—Anti-T Mist Revitalize and smoothens skin.
Europe countries (UK, Italy, Spain, Netherlands)
Bioearth—Siero Idratante Lenitivo Soothes skin.
Cantabria Labs Biretix—Gel Ultra
Purifying
Hydrating and soothing activity.
Dermalex—Rosacea Treatment Relieve different skin conditions.
Biodermal—Couperose Crème Soothes skin.
ITreatSkin—Neem Cream
Soothes and reduces skin
Inflammation.
Table 3. (Continued)

17
Figure 4. products containing
Boswellia Serrata
.resin extracts

18
1.2. Antioxidants
It serves as a protection mechanism to shield the body's cells from harm. The body's
enzymes and some nutrients consumed in everyday food make up antioxidants. Free
radicals lose their capacity to oxidize after they form, become resistant, and change into
another form. A free radical is an atom or molecule with one electron in the outer
orbit. This forces it to seek out the lost electrons from other body compounds, damag-
ing the body's cells by rupturing the barrier surrounding them. It does this by interact-
ing with the phospholipids in cell membranes, which causes damage to everything from
DNA to the collagen layer of the skin.
Antioxidant proteins protect cells from potential damage caused by free radicals.
Accurate identification of proteins and understanding their role in antioxidant activity is
critical in contributing to delayed aging[37]
1.2.1. Definition
Oxidation is a chemical reaction, in an oxidation process hydrogen or a material
transfers its electrons to an oxidizing agent. Oxidation can generate free radicals.
processes. These radicals can spark subsequent chain reactions. A cell may sustain harm
or even perish when the chain reaction takes place inside of it. Additionally, oxidative
stress both causes and results in illness. Protein molecules known as antioxidants stop
these chain reactions by eradicating the free radical intermediates and prevent additional
oxidation processes. Antioxidants are frequently reducing substances like thiols, ascorbic
acid, or polyphenols because they are oxidized themselves in order to accomplish
this[38] The definition can be summarized as that an antioxidant is "Any chemical that
significantly slows down or prevents the oxidation of a substrate when present in con-
centrations below those of the substrate" Both compounds with an enzymatic and
non-enzymatic nature are covered by this classification. Naturally, the diversity of anti-
oxidants must correspond to that of oxidants[38]

19
1.2.2. Free Radicals and Oxidative Stress
Extrinsic damage to the skin arises from various causes: Ionizing radiation, high lev-
els of stress on physical and emotional, alcohol use, poor diet, overeating, environ-
mental pollution, and UV radiation exposure (UVR) (Figure4) when the formation of
ROS in the skin caused by UV exposure surpasses the target cell's antioxidant defense
capacity, oxidative stress results[39] Acute UVR exposure reduces the activity of the
catalase enzyme in the epidermis and elevates protein oxidation[40]
According to estimates, UVR contributes up to 80% of all environmental factors,
making it the primary environmental factor influencing the occurrence of skin cancer
and skin aging[41] UVR causes molecular reactions in the human epidermis primarily
through the photochemical production of ROS, particularly hydroxyl radical, singlet oxy-
gen, superoxide anion, and hydrogen peroxide (H2O2)[42] UVR passes through the
skin, enters the cells, and interacts with DNA to cause the creation of photoproducts
that render DNA inactive. There are two distinct ways that UVR might harm an organ-
ism: (a) the cellular components directly absorb the incident light, creating excited
states that trigger a chemical reaction; and (b) pathways for photosensitization, in which
light is absorbed by endogenous (or exogenous) sensitizers that have been excited to
their triplet states. Two methods exist by which the energized photosensitizers can harm
cells: (a) the production of free radicals through methods for hydrogen abstraction and
electron transport, or (b) the production of singlet oxygen through energy transfer with
oxygen[43]
1.2.3. Skin Antioxidants
Antioxidants serve as a network of defense for the epidermis. They include enzy-
matic antioxidants like glutathione peroxidase, superoxide dismutase, and catalase, as
well as nonenzymatic low-molecular-weight antioxidants such as different forms of vita-
min E, vitamin C, glutathione (GSH), uric acid, and ubiquinol.[44] The outer layer of
the skin, known as the epidermis, contains a higher concentration of antioxidants than
the dermis. Potent antioxidants include ascorbate, carotenoids, and sulphydrils, which are

20
also abundant in the epidermis. The water-soluble antioxidants glutathione, glucose, pyr-
uvate, uric acid, ascorbic acid, and bilirubin are all present in plasma. Ubiquinol-10, ly-
copene, -carotene, lutein, zeaxanthin, and alpha-carotene are lipid-soluble antioxidants
similar to alpha-tocopherol[45] The most noticeable antioxidant in the lipophilic phase is
α-tocophero, while the cytosol contains the greatest concentrations of vitamin C and
GSH. According to molar ratios, hydrophilic non-enzymatic antioxidants like GSH, uric
acid, and L-ascorbic acid appear to be the most abundant antioxidants in human epi-
dermis[46] It was discovered that the stratum corneum (SC) contains both hydrophilic
and lipophilic antioxidants. The SC was discovered to contain GSH, uric acid, and vita-
mins C and E (both αγ and α-tocopherol)[47]

21
Figure 5. Environmental causes causing the formation of free radicals.

22
1.3. Antimicrobial
Threats from bacterial diseases to human health have been on the rise. Numerous an-
ti-infectious agents have been developed to efficiently reduce bacterial contamination
due to advances in biological technology and general hygiene. Antibiotics have been
widely used to combat bacteria and have successfully treated many illnesses. However,
one of the biggest threats to public health continues to be the emergence of drug
resistance from inappropriate antibiotic use.
A substance known as an antibiotic prevents the growth of (bacteriostatic agent) or
kills microorganisms (microbicide)
Antimicrobial drugs can be categorized based on the microbes they are most effective
against. For example, antibiotics are used against bacteria, while antifungals are used
against fungi. They can also be grouped according to how they are put to use. The use
of antimicrobial drugs to treat and prevent infections is referred to as antimicrobial pro-
phylaxis and antimicrobial chemotherapy.
1.3.1. Defense systems of the skin
The epidermis is a barrier to stop the spread and invasion of dangerous germs.
Among the cutaneous antimicrobial defense mechanisms are mechanical rigidity of the
stratum corneum, low moisture content, lysozyme production, acidity (pH 5), and de-
fensin[48] Generally, the skin's surface areas are dry, preventing bacterial growth.
Colonizing bacteria are eliminated via the sloughing of dead keratinocytes. Skin is cool-
er than normal body temperature and slightly acidic; most bacteria survive best at a
neutral pH and temperature of 37°C. If organisms get beyond human cutaneous de-
fenses, the immune system or skin-associated lymphoid tissue (SALT) steps in as the
next line of defense[49]

23
1.3.2. Bacterial growth on skin
Numerous bacteria have a complex habitat on the epidermis. The human epidermis is
initially sterile, but it quickly becomes a host to residing bacteria after birth. The varie-
ty and density of bacteria are influenced by anatomical location, ambient humidity, the
quantity of sebum and sweat produced, the host's hormonal condition, and age[50] In
relation to the host, the bacterial epidermis flora is parasitic, symbiotic, or commensal.
Although changes the type of interaction created is frequently intrinsic to the bacteria,
even if the host's immunological condition is recognized to have a considerable
influence. The ability of germs to stick to skin epithelium and thrive in mostly dry en-
vironments, and acidic environment, and then quickly read here during the usual des-
quamation process leads to persistent colonization[51]
1.3.3. Protection by colonizing bacteria
Commensal bacteria can grow on the skin, which helps to both directly and in-
directly safeguard the host from pathogenic bacteria. The production of bacteriocins, the
creation of toxic byproducts, the formation of minimal potential for reduction-oxidation,
the depletion of vital nutrients, the prevention of the adhesion of rival bacteria, the
Toxins' breakdown and blockage of translocation are just a few examples of the direct
effects. competition between commensal microbes for resources, niches, and receptors.
For instance, Staphylococcus epidermidis binds to keratinocyte receptors and prevents
pathogenic S. aureus from adhering to the skin[52] Commensals have the ability to pro-
duce bacteriocins, which are species-specific antibiotics. As an illustration, the S. aureus
strain 502A produces bacteriocins that prevent the growth of other virulent staph-
ylococcal pathogens[53] Indirectly, microbes can cause the host to produce more inter-
feron, cytokines, and phagocytosis, as well as more antibodies, phagocytosis, and clear-
ance mechanisms. For instance, Propionibacterium acnes releases fatty acids from lipid
decomposition, which acidifies the environment and stops Streptococcus pyogenes from
growing[54]

24
1.3.4. Microbes that cause skin diseases
Skin acts as a protection barrier and is home to numerous colonizing microorganisms.
Generations of dermatologists have claimed that microbes affect the natural course of
various skin conditions based on study and clinical observation. For instance,
Staphylococcus epidermidis is commonly cultivated from healthy skin and may shield
people from pathogenic bacteria[55] The most frequent bacterial skin and skin structure
infections are Scarlet fever, acute paronychia, staphylococcal scalded skin syndrome, cel-
lulitis, erysipelas, folliculitis, furunculosis, carbuncles, wound infections, abscesses, and
cellulitis[56] Bacteria are only one aspect of the relationship between microbes and hu-
man epidermis. Athlete's foot and chickenpox are just two examples of the well-known
human infections caused by fungi and viruses[57] a wide range of additional factors
may influence the microbial communities living on and in the epidermis. External ele-
ments including clothing types, use of lotions/creams, cleansers, deodorants, or anti-
perspirants, regularity of personal hygiene, seasonal temperature, ambient humidity, past
antibiotic use, and other environmental surfaces[58]
Microbes can cause skin infections. A wide variety of microorganisms can cause skin
infections. Most cases are brought on by streptococcus or staphylococcus aureus can be
treated with topical antibacterial. Table 3

25
Infections
Microorganisms Description Ref.
Nonbullous
Impetigo
staphylococcus
aureus
Lesions of nonbullous impetigo
typically begin on skin of the
face or extremities. a small vesicle
or pustule first appears, then
quickly transforms into a hon-
ey-colored, crusted plaque with a
diameter typically less than 2
centimeters. Lesions are typically
accompanied by little to no pain;
Pruritis occurs occasionally, and in
up to 90% of cases, regional ad-
enopathy is discovered.
[59-61]
B u l l o u s
Impetigo
staphylococcus
aureus
Most frequently, skin on the
cheeks, buttocks, trunk, perineum,
and extremities develops flaccid,
transparent bumps. Bullae readily
rupture, leaving a thin scale rim
at the edge of a moist, shallow
erosion.
[60, 62]
Table 4. The most common microbial infection

26
Infections
Microorganisms Description Ref.
Erysipelas
Group A be-
t a - h e m o l y t i c
S t r e p t o c o cc u s
p y o g e n e s
(GABHS)
Small erythematous patch with
well-defined, slightly raised bor-
ders that quickly turn brilliant red,
edematous, indurated, and shiny. It
is most commonly seen on the
central face and legs. The in-
fection spreads quickly, unevenly,
and laterally over a short period,
and it has the potential to worsen
into an infection with the develop-
ment of bullae and severe
necrosis.
[63]
cellulitis
s t r e p t o c o c c i
and
S
.
aureus
Hemorrhagic cellulitis may re-
sult from petechiae and ecchy-
moses, with numerous bullae de-
veloping on inflamed skin. are
rapidly spreading skin diseases
that are more severe than er-
ysipelas and affect the subcuta-
neous tissues. Regional lympha-
denopathy and lymphatic streaking
associated with the condition are
intermittent, and local con-
sequences like necrosis and ab-
scesses are more common than in
erysipelas.
[64]

27
1.3.5. Antibacterial
Antibacterial activity is linked to substances that kill or retard the development of
bacteria locally while not generally being toxic to nearby tissue. The majority of mod-
ern antibiotics are natural substances that have been chemically altered[65] The agents
can commonly fall into two categories: bacteriostatic, which inhibits bacterial develop-
ment, and bactericidal, which kills bacteria. Antibacterial medications are crucial in the
battle against infectious diseases. However, due to their widespread use and abuse, bac-
terial resistance to antibacterial drugs is now a regular occurrence, which is a sig-
nificant issue. Resistance is typically founded on evolutionary processes that happen
during, for example, antibiotic therapy and can be passed down inheritably.
Additionally, tolerance may develop through horizontal gene transfer via conjugation,
transduction, or transformation. [66]

28
1.4. Research Trends and Composition
1.4.1. Research Trends
Due to the tight relationship between human life and plant life as food, bot-
any research and identification are crucial. In addition to using plants as food, people
have linked the diseases they suffer from to the plants that cover the earth's surface
and have used these plants or parts of them as medicines.
Since the discovery of the advantages of plant extracts until now, many developed
nations have turned their attention to the significance of plant extracts, and the pharma-
ceutical and biological industries are flourishing as a result[67] With the discovery of
pure and efficient plant compounds in the nineteenth century due to the development of
chemical sciences and laboratories, the use of natural extracts continued and became a
significant part of molecular sciences. This led to the identification and extraction of
many compounds, such as polyphenols, and the importance of plant compounds that
have medicinal properties.
In America, 25% of medicines are herbal, an environmentally friendly product
free of synthetic chemicals is now widely accepted by people[68]
Additionally, the majority of the plant includes one or more chemicals, either nat-
urally or through active chemicals extracted from it, in small or large concentrations
that can treat one or more specific diseases or lessen their symptoms.
The beauty field is seeing the emergence of a new business in addition to the
pharmaceutical one: "green cosmetics." Since 2007, the market for natural and organic
skin and body care items has increased by an average of 6% annually, reaching over
$50 billion[69]
Since the beginning of time, people have been preoccupied with beauty and out-
ward looks. As a result of numerous bothersome external factors, this leads to the de-
velopment of melasma, wrinkles, hair loss or unwelcome hair growth, and skin diseases.
Male or female, people were eager to find solutions to these issues and to present
themselves in the best and most attractive way possible, so they took care of their

29
skin, its freshness, the length of their hair, its luster, and everything else related to
beauty[70]
Different kinds of cosmetics emerged over time and became a necessity in the
lives of the majority of people as human nature and living circumstances improved.
The types and uses of cosmetics differ, and among the best cosmetics that are
significantly superior to other preparations are those made from only naturally occurring
materials. It has a negative impact on the face, and many allergic individuals have dis-
covered that natural products, whether in the form of oils, creams, or other for-
mulations, work best for these issues.
Natural products play a part in all aspects of the body's health and beauty, not
just in medical treatments. Natural extracts and essential oils can whiten and color-coor-
dinate skin, remove excess hair, lengthen hair and smooth feet, and provide a variety
of other health advantages.
In light of this, natural and organic cosmetics are still in high demand from con-
sumers who have been influenced by recent media and social media posts providing
facts about green cosmetics, who produces them, how they are better for your skin
than conventional cosmetics, and the health risks of traditional colorants and poses.
Health-conscious consumers purchase these products to limit their exposure to poten-
tially harmful chemicals like parabens, and they demand that businesses be transparent
about the ingredients used in the products they use on a daily basis. They also discuss
what is required to create a product that falls into the category of eco-friendly prod-
ucts[71]
Manufacturers and business owners were under much pressure due to all these
customer expectations and demands, so they began spending much money to encourage
scientists and researchers to find natural-source compounds for use in developing new,
marketable products[72]

30
1.4.2. Structure of the study
Boswellia Serrata
resin are one of the primary resin used in this field because their
chemical compounds have beneficial effects on the body, such as their anti-in-
flammatory, anti-bacterial and antioxidant effects. Therefore, this study aimed to find the
best way to extract antioxidants and antimicrobial from
Boswellia Serrata
resin.
Boswellia Serrata
is one of the considered one of the most important frankincense
found in the homes of Arabs, Middle Easterners. Families trust this plant for its safe
and practical uses. Recent studies demonstrated that boswellic acids and
B
.
serrata
ex-
tract have a significant safety margin According to many studies, it has also been re-
ported for antioxidant, antimicrobial activity, which is attributed to compounds with free
radical scavenging activity due to its phenolic content. The
Boswellia Serrata
resin
extract generally contains boswellic acids, Fatty acids, and bioactive components.
Therefore, the experiment was designed to antioxidant and antimicrobial properties
Boswellia Serrata
resin extract and assessment of the ability to root scavenging using
DPPH and ABTS assay and measurement of phenolic components TPC and TFC. be-
side the experiment of HPLC analysis to confirm the polyphenols present in the
extract.
In addition to containing effective antimicrobial materials, a test was carried out
Antibacterial agar disc diffusion assay.
The purpose of this study is to investigate the possibility of using Boswellia Serrata
resin extract as functional ingredients of cosmetics by studying the results of root
scavenging and antibacterial tests according to the extraction method.

31
Ⅱ. Literally review
2.1. Review of research on
Boswellia serrata
2.1.1. Ingredients and compounds of
Boswellia serrata
The polysaccharides in oleo gum-resins (65% arabinose, galactose, and xylose) are
soluble in water. The resins (30–60%) and essential oils (5%–10%) are soluble in or-
ganic solvents. The resinous part of Boswellia serrata possesses monoterpenes, di-
terpenes, triterpenes, tetracyclic triterpenic acids and four major pentacyclic triterpenic
acids i.e. β-boswellic acid, acetyl-β-boswellic acid, 11-keto-β-boswellic acid and ace-
tyl-11-keto-β-boswellic acid, responsible for inhibition of pro-inflammatory enzymes.
One of these four boswellic acids, acetyl-11-keto-boswellic acid, is the most potent in-
flammatory enzyme inhibitor. 5-lipoxygenase[22]
According to a phytochemical analysis using According to thin-layer chromatography,
terpenoids, phenolic compounds, flavonoids, and phenylpropanoids were the major con-
stituents of B. serrata's gum resin[34]
a. Resin \ Gum
Oleo-gum resin analysis revealed: Moisture 10-11%, volatile oils 8-9%, resins
55-57%, gums 20-23%, and insoluble materials 4% - 5%.
The techniques for separating oleo gum resin into its various components and gum
enzymes like diastase and oxidase have also been studied. The gum has 0.16% nitrogen
in it by[73] The gum was hydrolyzed by heating it with 3% H2SO4 recognized the
sugars as arabinose, xylose, and galactose after 8 hours by [74] Comparable to those
made with 5% Acacia mucilage were tablets made with 9% B. serrata mucilage. identi-
fied water soluble protein of gum resin, 4-O-methyl-glucuronoarabinogalactan[25]
Boswellia serrata gum resin extracts are effective at treating several inflammatory dis-
eases, include asthma, rheumatoid arthritis, osteoarthritis, inflammatory bowel disease de-
pending on animal research and preliminary clinical trials[75]

32
b. Oil
A-thujene, a-pinenes, a significant ingredient, and b-phellandrene were discovered in
minute quantities in the low boiling oil fractions. Terpenol, methyl chavicol, and sesqui-
terpenes were the three main constituents that worked out the high boiling fractions in
detail. It has also been claimed that acetyl-b-boswellic acid separation was accomplished
using spectral data and interconversion. The qualities and applications of essential oils,
as well as the methods for separating them from gum and resin.
Due to its diverse sources, oil has a wide range of physicochemical properties.
A-pinene dipentene, phellendrene, cadinene, camphene, p-cymene, d-borneol, verbenone,
and verbenol are some of the components of the oil. According to numerous research,
the principal components of the essential oil include a-thujene (50%) a-pinene (6.2%),
dlimonene (4.5%), p-cymene (14%), cadinene (4%), geraniol (0.8%), and elemol (1.3%).
The main components are the a and b-pinenes and d-emonene. Terpinyl acetate 3.5%,
methyl chavical 2%, linalool 1.5%, and terpinol 1% are present.[25]
c. Boswellic acids
boswellic acids in particular have been found to be active constituents; their chemical
structures are shown in (Figure5)
The content of six boswellic acids [keto boswellic acid (1), 3-O-Acetyl 11-keto β
-boswellic acid (2), α-Boswellic acid (3), β-Boswellic acid (4), 3-O-Acetyl-ɑ-boswellic
acid (5) and 3-O-Acetyl-β-boswellic acid (6)] had been used as the standard index to
appraise the quality of boswellia gum resin and its products[76]
In 11-keto BAs (KBA) and 3-O-acetyl-BAs (AKBA), respectively, a carbonyl and
acetyl groups are present, providing structural variation[77] The main anti-inflammatory
properties of boswellic acids are attributed to suppression of leukotriene formation via
inhibition of 5-lipoxygenase (5-LO) by 11-keto BAs (KBA) and 3-O-acetyl-BAs
(AKBA)[75]
In addition to inhibiting human leukocyte elastase, which is produced in inflammatory

33
and hypersensitive conditions, boswellic acids also have antiphlogistic effects[78]
d. Terpenoids
On the basis of b-boswellic acid, which makes up more than 30% of the total tri-
terpene acids, the estimation of the total triterpene acids present in the various forms of
B. serrata was made. B-boswellic, 11 ketoboswellic, and acetyl 11-keto b-boswellic
acids are all types of triterpene acids. Utilizing functional groups analysis, triterpene
acids were estimated individually or in conjunction with one another. Acetyl and hy-
droxyl groups at position 3 and keto groups at position 11 were the functional groups
examined[25]
e. Fatty acids
The Boswellia serrata tree's bark contains Fatty acids like Myristate, Palmatic, Oleic,
Linolic, Arachidate, Arachidatenate, and Lignocerate. It was discovered using a
GLC-MAS tool, which has proven to be effective against bacteria[79, 80] The GLC
technique was used to identify the fatty acids in the extract of frankincense resin. The
equipment produced GLC-chromatograms and extractor retention times that were com-
pared to standard chemicals. Each fatty acid's concentration in the extract was also
known. The following fatty acids were identified: (Myristate, Palmatic, Oleic, Linolic,
Arachidate, Arachidatenate, and Lignocerate). From highest concentration to lowest con-
centration, the fatty acids identified with the GLC device are as follows: (Palmatic
0.129, Myristate 0.119, Lignocerate 0.114, Lenolic 0.026, Arachidatenate 0.008, Oleic
0.007). Additionally, it displayed each fatty acid's concentration and retention period af-
ter being separated from olibanum. According to the study's by [80] Olic acid was
found to be present in the lowest percentage 0.007 and palmatic acid in the greatest
percentage 0.129 followed by the remaining separated fatty acids. Numerous studies
have established that the Boswellia serrata plant includes a variety of fatty acids, in-
cluding Oleic, Linoleic, Arachidic, Arachidonic, Palmitic, and Lauric acids[17]

34
f. Phenols
The Boswellia serrata tree resin demonstrated that includes phenolic chemicals that
have demonstrated their effectiveness as antibacterials, including thujene, camphene,
b-pinene, myrcene, limonene, and cis-verbenol.by [34]
Previous studies have proven that Due to its anti-inflammatory properties, the frank-
incense resin of the Boswellia serrata plant, which contains free phenols, is used in the
treatment of many human illnesses, particularly reducing the symptoms of arthritis.
Additionally, the Boswellia serrata extract included free phenols in a variety of concen-
trations, and these had a somewhat stronger impact than the fatty acid extract on patho-
genic microbes[17]

35
Figure 6. Chemical structures of boswellic acids.

36
2.1.2. Bioactive compounds in Boswellia serrata
The Boswellia serrata plant contains gum and essential oil. Monoterpenes, diterpenes,
and sesquiterpenes are all present in their essential oil. Essential oils also contain phe-
nolic substances and diterpene alcohol(serratol). The drug's gum component comprises
Pentose and Hexose sugars and certain oxidizing and digesting enzymes. The major
component of the resin is pentacyclic triterpene acid, whose active moiety is boswellic
acid.[81] NMR and mass spectroscopy were used to corroborate the structure of the
fraction produced 3-hydroxy-lup 20(29) ene-24-oic acid following further purification
with EtoAC- Hexane (1:1)[82] B. serrata gum-resin samples from India and Africa that
underwent HPLC analysis produced 12 different pentacyclic triterpene acids[21]
Pinene and cymene were discovered through thin layer chromatography (TLC) exami-
nation of the essential oil from B. serrata leaves using silica gel and spraying reagents
with vanillin-sulphuric acid. Their Rf values were 85 and 33, respectively. While GLC
tests using OV-17 and SE-30 at 69-200°C produced thirteen components, including
d--thujene (32%), p-cymene, and d-limonene as minor constituents in the lower boiling
fraction, and -terpineol, methyl chavicol, and four unidentified compounds in the high
boiling fraction[83] Using a highly sensitive reverse phase HPLC technology, boswellic
acids in Boswellia serrata were found and analyzed was used at 210 and 254 nm with
an acidic mobile phase at 60oC[84]
Boswellia serrata n-hexane extract was steam distilled, and GC-MS analysis of the
essential oil fraction revealed 33 components[21] comprising monoterpenes (9.9%) and
diterpenes (7.1%), esters (62.1%), and alcohol (15.4%). This essential oil was dis-
covered to contain the following compounds: α-thujene, α-pinene, camphene, sabinene,
β-pinene, myrcene, o-methylanisole, αterpinene, hexyl acetate, p-cymene, 1-8-cineole, li-
monene, cis-β-ocimene, trans-β-ocimene, γ-terpinene, 1-octanol, terpinolene, linalool,
1-decanol, terpinen-4-ol, α-terpineol, 1- octylacetate, bornyl acetate, citronellyl acetate,
neryl acetate, geranyl acetate, hexyl hexanoate, 1-decyl acetate, hexyl octanoate, iso-
cembrene, cembrene, iso-incensole and incensole[85] By employing column chromatog-
raphy with silica gel-G with n-hexane and ethyl acetat, the tetracyclic triterpene acids
E, F, G, and H from resin of Boswellia serrata were obtained[21]

37
Boswellia serrata oleoresin was successfully adapted for determine using solid phase
microextraction and gas chromatography/mass spectrometry the volatility and polarity of
terpenoids. As a consequence, cembrane and incensole were captured as unique
diterpenes. Additionally, 50 monoterpenes were obtained by gas chromatography at 40°C
using poly-dimethylsiloxane/divinylbenzene fiber, with roughly 15 of them having a
yield of more than 1%. after trimethylation yielded 15 triterpenes i.e. α-boswellic acid,
β-boswellic acid, 3-acetyl-αboswellic acid, 3-acetyl-β-boswellic acid, α-amyrin, β-amyrin,
3-epi-αamyrin, 3-epi-β-amyrin, lupeol, 3-epi-lupeol, αamyrenone, β amyrenone, lupenone,
3α-hydroxy lup20(29)en-24 oic acid and 3-O-acetyl hydroxy lup-20(29) en-24-oic acid
on GC-MS studies. In addition, three distinctive degradation products were discovered:
24-norroleana-3,12-diene (a), 24-norursa-3,12-diene (b), and 24-norlupa-3,20(29)-diene
(c)[21] The key characteristics and chemical composition of B.serrata extracts are out-
lined in the table 3.

38
ingredients and compounds in
Boswellia serrata
Ref.
Galacturonic acid, glucose, galactose, fructose, sitosterol, phe-
nol-o-cresol, m-cresol, p-cresol, thymol, and carvacrol are all
sugars.The acid campholenic Campholytic acid and 2,2,4-trimethylcy-
clopent-3-en-1-yl acetic acid
[86]
percentage (97.3%) rich in limonene and e-ocimene. Sesquiterpene
content in E-caryophyllene is (2.7%).
[87]
Resin acids comprise between 60% to 70% of the mixture, together
with water-soluble gum (20%) and monoterpene essential oil
(3%–10%).
[88]
oil(45%), α-thujene (12%), α-pinene (8%), sabinene (2.2%),
β-pinene (0.7%), myrcene (3.8%), α-phellandrene (1%), pcymene
(1%), limonene (1.9%), linalool (0.9%), perillene (0.5%),
methylchavicol (11.6%), methyleugenol (2.1%), germacrene D (2.0%),
kessane (0.9%), cembrene A (0.5%) and cembrenol (1.9%), a
monoterpene 5,5-dimethyl-1-vinylbicyclo- hexane (2%) and
m-camphorene (0.7%) and p-camphorene (0.3%)
[89]
Diterpenes, incensole, incensole acetate, and cembrenol (serratol)
[90, 91]
Lupeolic acids, oleanane(α-boswellic acids), ursane-(β-boswellic
acids) , and lupane-type lipophilic pentacyclic triterpene acids, and an
ether-insoluble fraction polysaccharides (arabinose, galactose, and xy-
lose) soluble in water
[19, 92]
Table 5. Main ingredients and compounds in
Boswellia serrata
.

39
2.1.3.
Boswellia serrata
in cosmetology
Plants have been used in cosmetics since antiquity, and modern scientific research
continues to center on this topic. It is now possible to draw even more intricate images
because of advances in our knowledge of how plants and skin change over time. Plants
are intelligent beings that respond to their environment by creating different metabolites.
Applying phytomolecules to the skin impacts the skin's health and appearance through
interacting with skin cells. Numerous plants with the potential to enhance modern cos-
metic products have been identified through both physico-chemical research and ethno-
botanical studies[93]
Plants offer precious active substances for both therapeutic and aesthetic uses.
Humans have utilized products meant to improve skin issues and appearance for hun-
dreds of years, and these products have evolved into modern cosmetics. Our skin is a
physical barrier between ourselves and the outer world and protects us from danger.
Plants have been proven to create compounds that help soothe and protect the skin. In
addition, Modern cosmetics can adjust the skin's elastic qualities along with hydrating
the skin and minimizing redness[94]
The major fibers that make up the skin's extracellular matrix are collagen and elastin;
the former is in charge of tensile strength, and the latter is in charge of elasticity. The
aging process is accompanied by a decline in collagen and elastin production and
strength, which results in wrinkles[95] Additionally, the creation of the enzymes colla-
genase and elastase, which break down collagen and elastin is additionally responsible
for the intrinsic aging of the skin[96]
Several things can cause damage to the outer skin, including intense UV radiation
exposure, a poor diet, and physical and mental stress. Oxidative stress, which produces
free radicals, is brought on by the production of reactive oxygen species by the impact
of UV rays on the skin[41], And research has effectively demonstrated a clear link be-
tween free radicals and the onset of premature aging[97]
According to estimates, UV rays account for up to 80% of all environmental influen-
ces, making them the most significant environmental component in the development of

40
skin cancer and skin aging[41]
Some of the applied antioxidants have the ability to preventively block the negative
effects of free radicals, resulting in normal generation of the structural proteins of the
skin[97] Vitamins and antioxidants applied topically in cosmetics are thought to enhance
protection and maybe even undo harm by neutralizing free radicals[98]
Since the skin serves as the body's external barrier against the environment, it is at
the forefront of the fight against external influences to destroy free radicals. Free radi-
cals are unpaired electron compounds that are very reactive that cause damage to the
surrounding molecules and tissues. It is known that ultraviolet light and environmental
pollutants are among the initiators to cause free radicals[99]
Several studies conducted on boswellia serrata material reported the presence of
many compounds such as polyphenols and terpenoids, These terpenoids, which are
thought to be the most potent components of Boswellia resin, are mostly represented by
boswellic acids[100] The researchers discussed polyphenols' antioxidant properties.
Terpenoids have been shown to have antioxidant capacity, and research has shown that
they are highly active molecules that can help slow skin aging[101]
Since ancient times, the Arabs and Indians have used Boswellia serrata and consid-
ered it an essential plant in their culture for its cosmetic properties and countless bene-
fits for skin and hair health.
Due to its antioxidant, antibacterial and anti-inflammatory properties, it helps get rid
of the most common skin and hair problems.
It works on common skin problems like acne, blemish-prone skin, aging skin, dry
skin, etc., and is suitable for all skin types. Frankincense essential oil's anti-in-
flammatory and antibacterial properties make it beneficial for acne-prone skin. It offers
sebum for aged skin and soothes greasy, acne-prone skin. Additionally, it serves as a
natural tone, enhances skin tone, and hides pores. It is a strong astringent and works
wonders on the skin's wrinkles, fine lines, scars, and stretch marks. Additionally, frank-
incense essential oil encourages the production of new skin cells, keeps the skin supple,
and calms dry, chapped skin[102]

41
It helps to even out the skin tone by preventing or reducing the occurrence of age
spots, sun spots, and other spots. Additionally, it lessens skin inflammation and redness
and helps obtain a homogeneous skin tone. It is also used as a treatment for bruises
and sore sores. It is a powerful anti-wrinkle and anti-aging agent that treats various
skin conditions, including psoriasis and eczema, and helps reduce hair loss, also for
suppression of skin and nail infections[19] Moreover, Boswellia serrata extracts have
been shown to reduce redness and irritation of the skin, act as a soothing agent for
sensitive skin and prevent the appearance of red dots after hair removal, and help even
skin tone[103](Figure6)

42
Figure 7. The illustrated list of cosmetic applications of
Boswellia serrata
.

43
Compound name Chemical structure
Proof of efficacy as an ingredient
in cosmetics
Ref.
Gallic acid
Gallic acid's ability to func-
tion as a cosmetic ingredient is
complicated by its heat
instability. Gallic acid was cou-
pled with a peptide to combat
this problem. It was possible to
produce galloyl-RGD, a promis-
ing candidate for the cosmetic
ingredient.
[104
, 105]
Vanillic acid
Vanillic acid helps the skin
of humans become lighter and
less pigmented. Since its skin
penetration has been established
and the toxicity test has been
successful, its use on the skin
has expanded.
[106
, 107]
Protocatechuic acid
Protocatechuic acid has the
capacity to treat skin aging in 8
weeks, according to research re-
sults on human skin.
108,
109]
Table 6. Chemical composition of
Boswellia serrata
. resin and use as cosmetic in-
gredients

44
Compound name
Chemical structure
Proof of efficacy as an
ingredient in cosmetics
Ref.
Syringic acid
Syringic acid slows down the
aging process of cells by
blocking ultraviolet B. Syringic
acid's effects as an antioxidant
and anti-aging agent con-
sequently increased the survival
rate of cells damaged by ultra-
violet B, suggesting that it can
be used as a natural phy-
tochemical in cosmetics.
[110,
111]
Cinnamic acid
Derivatives of cinnamic acid
are frequently utilized as UV
protection, antioxidant, and anti-
bacterial agents in cosmetic
goods.
[112,
113]
Caffeic Acid
Caffeic acids attracted much
attention because they are
promising and unaffected by
free radical toxicity. Due to its
antioxidant properties, it is
found in cosmetic goods.
[114,
115]
Table 6. (Continued)

45
Compound name
Chemical structure
Proof of efficacy as an
ingredient in cosmetics
Ref.
Ferulic acid
Ferulic acid is a protein in-
hibitor that catalyzes the gen-
eration of free radicals and en-
hances the action of scavenger
proteins. The primary skin
structures are protected by
ferulic acid.
In addition to enhancing an-
giogenesis and promoting
wound healing, it inhibits
melanogenesis. It is typically
used to fight photoaging as a
skin-brightening component in
skincare products.
[116,
117]
Sinapic acid
Sinapic acid protects the skin
cell from complete collagen
degradation by preventing UVB
activation, reducing the in vivo
effects of photoaging, and re-
ducing skin tissue inflammation.
[118,
119]
Table 6. (Continued)

46
Compound name
Chemical structure
Proof of efficacy as an ingredient
in cosmetics
Ref.
Pelargonic acid
A fatty acid called pelargonic
acid can serve as a surfac-
tant-cleansing agent as well as a
fragrance ingredient and emulsi-
fier in cosmetic products. This
chemical is safe to use in cos-
metic products to improve skin
penetration, according to the
Cosmetic chemical Review
(CIR) Expert Panel's earlier
ruling.
[120
, 121]
p-Hydroxybenzoic
acid
p-hydroxybenzoic acid is uti-
lized in cosmetics to prevent the
growth of microorganisms and
extend the shelf life of cosmetic
and personal care goods.
[122
, 123]
Catechin
By crosslinking, catechin can
improve collagen arrangement.
Studies show that catechin mol-
ecules attach to collagen fibers.
[112
, 124]
Table 6. (Continued)

47
2.2. A review of research on
Boswellia Serrata
Boswellia Serrata
is an excellent plant, serving as medicine and prevention. Due to
this, many researchers in the health sciences have focused their attention on figuring
out the most effective ways to use this plant to both prevent and treat a variety of
ailments.
Boswellia serrata
is a plant with significant medicinal and cosmetic value.
Numerous studies, trials, and applications have demonstrated promising therapeutic
effects.
a. Toxicity and safety
Boswellia serrata
is considered one of the most important frankincense found in the
homes of Arabs, Middle Easterners. Families trust this plant for its safe and practical
uses. Recent studies demonstrated that boswellic acids and B. serrata extract have a
significant safety margin.
Boswellia gum resin, which has been used as a medicine for thousands of years, is
thought to be secure because it hasn't caused any serious side effects[125] Boswellia
has remarkably low toxicity, and unlike many chemical anti-inflammatory drugs, its an-
ti-inflammatory effects have no negative impacts on blood pressure, pulse rate, respira-
tion, or other autonomic responses[126] Boswellia gum resin has been authorized for
use as a food additive by the US Food and Drug Administration (USFDA), and it is
included on its list of safe substances[127] The markets are sell over-the-counter an-
ti-inflammatory products that are oral preparations of B. serrata extract containing
AKBA[127] In previous studies, it was observed that no deaths were noted following a
single dosage of B. serrata extract administered to mice at a dose of up to 5 g/kg[128]
Mice were administered the extract orally for 28 days in a row without showing any
behavioral toxicity, and their hepatic and renal function biomarkers did not significantly
change as a result[129] Boswellia extract was used in animal toxicology tests, and the
findings showed that it was safe to use in herbal remedies[76, 130]

48
b. anti-inflammatory
Boswellia serrata has been shown to be a strong anti-inflammatory medication in
both clinical investigations and in-vivo animal models. Boswellic acid inhibits leuko-
triene production in a dose-dependent manner by acting as a selective, non-redox in-
hibitor of 5-lipoxygenase. Additionally, it has been shown to reduce levels of leuko-
triene B4 and 5-hydroxyeicosatetraenoic acid, two active chemotactic agents responsible
for increased vascular permeability and pro-inflammatory 5-lipoxygenase products. As a
result, less white blood cells are drawn to the area of inflammation, which dampens the
inflammatory response and promotes speedier healing when boswellic acid is used as a
treatment.
Boswellic acid reduces primary antibody production, polymorphonuclear leukocyte in-
filtration, and migration and nearly completely inhibits the classical complement system.
When boswellic acid was studied in vitro for its impact on the complement system,
both the traditional and alternative pathways were significantly inhibited[131]
c. Hypoglycemic
A herbal supplement by modulating hepatic gluconeogenesis, pyruvate carboxylase,
and phosphoenol pyruvate carboxykinase, it has been shown to have considerable an-
ti-diabetic activity on non-insulin dependent diabetes mellitus in streptozocin-induced di-
abetic rats[21]
d. Analgesic and Psychopharmacological activity
Boswellia serrata's non-phenolic fraction exhibits sedative and analgesic properties.
Additionally, the potentiated enhanced secobarbitone-induced hypnosis in rats, with no
discernible effects on the conditioned avoidance response[21]

49
e. Muscle Relaxant activity
It was revealed that the essential oil of B. serrata has stimulatory effects on skeletal
muscles and spasmogenic effects on the smooth muscle of the guinea pig ileum.
According to a prior study, the B. serrata essential oil operates directly on biological
tissues and is not activated by non-specific cell membrane action[21]
f. Anti-Alzheimer’s activity
Alzheimer's disease (AD) is a chronic neurological illness. A common and early
symptom of AD is increased oxidative stress. Antioxidant-active medicinal plants have
long been used to treat various human illnesses. Boswellia may be able to treat
AlCl3-induced Alzheimer's by increasing Ach levels and lowering AchE activity in
brain homogenates, according to the study. A time-dependent improvement in de-
mentia-type AD caused by i.c.v. Streptozotocin injection has also been found to be pos-
sible with frankincense[132]
g. Antidepressant activity
The plant's extract is used in a variety of tea blends and as aromatherapy. According
to reports, B. serrata is effective in treating acute depression. Boswellia exhibits strong
antidepressant efficacy in acute stress trials and decreases the immobility time in the
experimental forced swim model at a dosage of 100 mg/kg. It was discovered that B.
serrata, a traditionally significant medicinal plant, acts as a bacteriostatic agent[132]

50
Ⅲ. Materials and Methods
3.1. Experimental Materials and Reagents
3.1.1. Materials
The devices used are a sensitive scale, Autoclave, inoculated Petri dishes, LM2 mill.
filter paper (Whatman No. 1), Rotarapor, electromagnetic, vibrating heater, Elisa reader,
freeze dryer, different glassware (cups, conical flasks, standard flasks of 500ml ca-
pacity), funnels, graduated condensers, glass stem, condenser, thermometer) and distilled
water.
3.1.2. Reagents
Reagents used for extraction in this study were ethanol (DUKSAN), methanol
(DUKSAN), and ethyl acetate (DUKSAN). For various physiological activity tests,
1,1-Diphenyl-2-picrylhydrazyl (DPPH, SIGMA), L -ascorbic acid (SIGMA),
2,2'-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS, SIGMA), folin-ciocalteus
phenol regent (SIGMA), potassium persulfate (SIGMA), KCl (DUKSAN) , NaCl
(DUKSAN), NaCO3 (DUKSAN), Na2HPO4 (DUKSAN), KH2PO4 (DUKSAN), gllic
acid (SIGMA), aluminum chloride (SIGMA), potassium acetate (SIGMA), quercetin
(SIGMA), kaempferol (SIGMA), isorhamnetin (SIGMA), quercetin dihydrate (WAKO),
6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (trolox, SIGMA), methanol
(THERMO FISHER SCIENTIFIC), phosphoric acid (DUKSAN) and ethanol (THERMO
FISHER SCIENTIFIC) were used. All reagents used for HPLC analysis were HPLC
grade reagents.

51
3.2. Extraction and Isolation
3.2.1. Sample collection
The oleo-gum-resin of
B
.
serrata
used was purchased from the Murshed Market in
Dubai, United Arab of Emirates. 500g of resin were collected and shipment to
Gwangju Korea Chosun University.
3.2.2. Extractration
3.2.2.1 Soxhlet Extraction
The Soxhlet Extraction was measured by modifying the method of S. Mishra[132] A
thimble containing around 5mg of the material was filled before being placed on the
Soxhlet extractor. Methanol (100mL) was then added to a distillation flask. The ex-
traction process was done for 6 h. After completing the Soxhlet extraction process, the
extract has been set in the concentration device Rotary Evaporator (Eyela N-1100,
Shanghai Eyela, China). When the extract dries, should add 40ml of methanol and put
it in an Ultrasonic device to combine the extract. Then the extract was divided into
several tubes, provided that the content of one tube did not exceed 20ml, and dried by
a Centra-Vac (VS-802, Vision, Korea) For up to a day. For the last step, place the ex-
tract in Freeze Dryer for a whole day. Calculation of Yield Extract B. Serrata 2.72g =
54.4% yielded.

52
Figure 8. Soxhlet Extraction diagram of
Boswellia Serrata
.

53
3.2.2.2 Immersion Extraction (70% EtOH)
200g of the sample (powder) was placed in the Ethanol 1400ml / Water 600ml was
add to the immersion container. 1 week immersion in mix in room temperature. After
completing the immersion process, The extract is filter with paper (Whatman No.
2)After filtering the extract has been set in the vacuum device and a (rotary vacuum
concentrator) When the extract dries, should add 90ml of Ethanol \ 10ml of Water and
put it in an Ultrasonic device to combine the extract. Then the extract was divided into
several tubes, provided that the content of one tube did not exceed 20ml, and dried by
a Centra-Vac (VS-802, Vision, Korea) For up to a day. Calculation of Yield Extract
B. Serrata 59.717g = 29.86% yielded
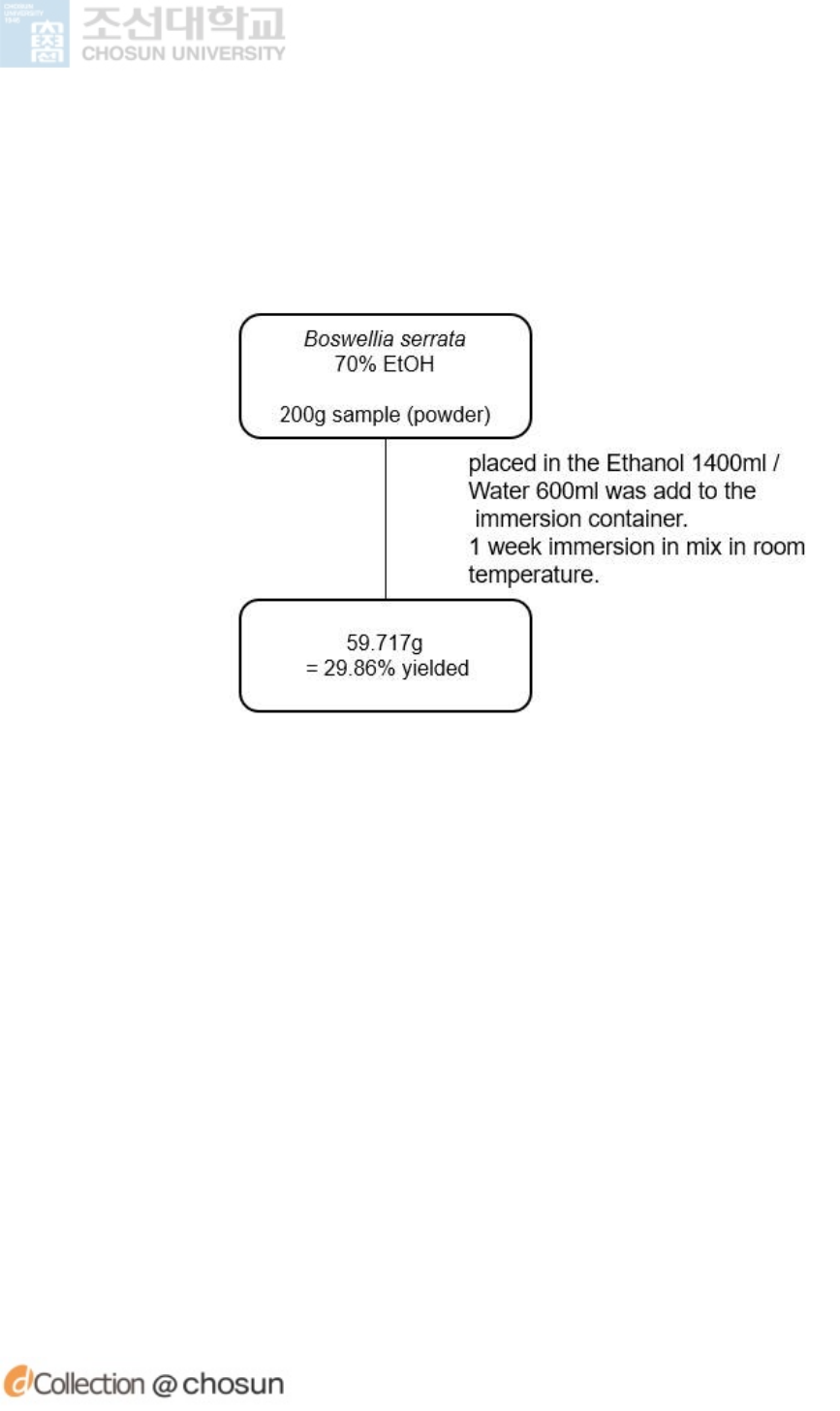
54
Figure 9. Immersion Extraction diagram of
Boswellia Serrata
.

55
3.2.3. floor separation
Place 300 mL of the solution (
B
.
serrata
70% EtOH 29.36g solvent with 100%
MeOH) in a clean separatory funnel and extract with 600 mL of Hexane x3 times then
300 mL of Water with 600 mL of Ethyl acetate x3 times
The separatory funnel should be stopped, Gently shake the funnel and flip it over.
Open the stopcock while the funnel is in this position to relieve the internal pressure.
Releasing the internal pressure once more after closing the stopcock and giving the fun-
nel a brisk shake This process should be repeated four or five times. Remove the stop-
per, place the separatory funnel upright in the support ring, and allow it to stand still.
Collect the lower aqueous layer of the liquids after they have separated, being cautious
not to let any of the top layer leak through the tap. Run the top layer through the
separatory funnel's tap and into a different conical beaker.

56
3.3. Antibacterial activity
3.3.1. Antibacterial agar disc diffusion assay
The Antibacterial agar disc diffusion assay was measured by modifying the method
of B. Chand [133] The in vitro antibacterial activity of the
B
.
Serrata
total crude ex-
tract and fractions was evaluated via the disc diffusion method with
S
.
epidermidis
,
M
.
furfur
,
M
.
pachydermatis
and the Anaerobic jar
C
.
acnes
using Tyrptic Soy
Agar(TSA), Modified Leeming Notman Agar(MLNA), Sabouraud Dextrose Agar(SDA),
and Reinforced Clostridial Agar(RCA) with determination of inhibition zones diameter
measured in millimeter (mm). Sterile filter paper discs (8 mm) were impregnated with
Concentration 3mg, 4mg, 5mg of
B
.
Serrata
extract and then placed on inoculated
Petri dishes containing bacterial suspension. Ampicillin disc (10mg) were used as pos-
itive control whereas discs without samples (10% DMSO (dimethyl sulfoxide) acted as
negative control. The zones of inhibition including the diameter of the extract impreg-
nated dics were compared with those of the controls after incubation.
S
.
epidermidis
at 37°C for 24h,
M
.
furfu
r at 30~37°C for 3~days,
M
.
pachydermatis
at 30~37°C
for 2~3days and the Anaerobic jar
C
.
acnes
at 37°C for 3days The inhibition zone di-
ameter (IZD) was used as criteria for the definition of active or inactive sponge
extracts. The tests were carried out in triplicate for each extract.

57
3.4. Antioxidant activity
3.4.1. DPPH free radical scavenging assay
The DPPH radical scavenging ability was measured by modifying the method of HP.
Singh [134] Mix 500 μL of 0.123 mM DPPH reagent with 500 μL of each Fraction
and each concentration of Boswellia serrata resin extract solution (500:500 DPPH)
Similarly, each concentration of standard Gallic acid 200 μL : 800 μL was treated with
DPPH reagent 0.5 μL to use as positive control, and after reacting in the dark for 15
minutes, Biotek Absorbance, was measured at 517 nm wavelength using Synergy HT
multi-detection microplate reader equipment. A comparative experiment was conducted
using gallic acid as a positive control group. The radical scavenging ability was
calculated by the following equation and expressed as a percentage. Each reaction was
measured in triplicate
% of scavenging activity = (A control – A sample) / A control × 100

58
Figure 10. Measurement of the activity of an antioxidant by the DPPH assay.

59
3.4.2. ABTS radical scavenging assay
ABTS radical scavenging ability was measured by modifying the method of Gupta
[135] Prepare 2,2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt
(ABTS, 14 mM) at a concentration of 7 mM and potassium persulfate (4.9 mM) at a
concentration of 2.45 mM to 1: After mixing in a ratio of 1. This solution was diluted
with phosphate-buffered saline (0.1 M, pH 7.4), value at 730 After mixing 1,000 μL of
ABTS solution with 200 μL of extracts of Boswellia Serrata resin extracts for each
Fraction and each concentration and reacting for 30 minutes, absorbance was measured
at 730 nm wavelength using Biotek Synergy HT multi-detection microplate reader
equipment. A comparative experiment was performed using quercetin as a positive
control. The radical scavenging ability was calculated by the following formula and ex-
pressed as a percentage. Each reaction was measured in triplicate.% of scavenging ac-
tivity = (A control – A sample) / A control × 100.

60
Figure 11. Measurement of the activity of an antioxidant by the ABTS assay.

61
3.5. Total polyphenol and flavonoid contents
3.5.1. Determination of total polyphenol content
Total polyphenol content was measured by modifying method of MA Ayub [34]
500 µL of 0.2 M Folin-Ciocalteu's phenol reagent and 500 µL of 2% sodium carbonate
aqueous solution (w/v) were mixed with 500 µL of extract solution of Boswellia
Serrata resin for each Fraction and each concentration and reacted for 40 minutes.
Absorbance was measured at 750 nm wavelength using Biotek Synergy HT
multi-detection microplate reader equipment. The final concentration of the extract was
10 ⅿg/mL, and the total polyphenol content was expressed as gallic acid (GAE) and
P-coumaric Acid mg/g equivalent based on the calibration curve.

62
3.5.2. Determination of total flavonoid content
Total flavonoid content was measured by modifying method of MA Ayub [34] After
adding methanol 750 µL, potassium acetate 50 µL, aluminum chloride 50 µL, and etha-
nol 1.4 mL to 250 µL of extract solution of Boswellia Serrata for each Fraction and
each concentration, react at room temperature for 40 minutes.
Then Biotek Synergy HT Absorbance was measured at a wavelength of 415 nm using
a multi-detection microplate reader. The final concentration of the extract was 500
ⅿg/mL, and the total flavonoid content was expressed as quercetin (QUE) and Rutin
mg/g equivalent based on a calibration curve.

63
3.6. Analysis of polyphenol componds
3.6.1 High performance liquid chromatography (HPLC) analysis
The polyphenols contained in Boswellia serrata extracts were quantitatively analyzed
by HPLC (SPD-20A, SHIMADZUCO., Japan). Gallic acid, catechin, (-)-epicatechin, val-
lic acid, narigin, ethyl gallate, p-coumaric acid, ferulic acid, benzoic acid, quercetin,
narigenin, kaempferol, and 4-hydroxybenzoic acid were used as polyphenol standards.
Standards were tested at a concentration of 100 μg/mL, and extracts were prepared at a
concentration of 1,000 μg/mL and filtered through a 0.45 μm syringe filter. The column
was analyzed using a Shimpack GIS-ODS (C18, 4.6 × 250 mm, 5.0 μm, Shimadzu
Co., Japan) with a flow rate of 1.0 mL/min and an injection volume of 10 μL. The
mobile phases were water (A, in 0.1% phosphoric acid) and acetonitrile (B). The gra-
dient conditions of the mobile phase were 0-10 min: B (8-35%), 10-15 min: B
(35-35%), 15-25 min: B (35-65%), 25-30 min: B (65-65%), 30-35 min: B (65-100%),
35-40 min: B (100-100%) based on the mobile phase (B), and the wavelength was
measured at 280 nm.

64
3.6.2 HPLC-MS/MS Analysis Method
In order to identify the active ingredients of goldenrod licorice extract, 24 poly-
phenols were analyzed by HPLC-MS/MS (AB SCIEX 4000 Q Trap LC/MS/MS System,
Shimadzu LC 20A System). A 10 μL aliquot of the sample was analyzed using a C18
column (Gemini 3 μm, C18 110A 50 mm*2.0 mm) at 40°C in a column oven and
15°C in an autosmpler. Anionic and cationic modes were analyzed using Turbo Ion
Spray, and water (0.1% formic acid) (A) and acetonitrile (0.1% formic acid) (B) were
used as mobile phases. The anionic mode was based on the mobile phase (B):
0-0.5min: B (20-20%), 0.5-2min: B (20-80%), 2-2.5min: B (80-80%), 2.5-2.6min: B
(80-20%), 2. 6-6min : B (20-20%) and 0-3min : B (30-30%), and the cationic mode
was analyzed as 0-0.2min : B (30-60%), 0.2-2min : B (60-60%), 2-2.1min : B
(60-30%), and 2.1-4min : B (30-30%).

65
Ⅲ. Results and Discussion
4.1. Yield of extract according to Immerion extraction method
The extraction of natural products also shows differences in the extraction yield
because the components differ according to the solvent Using Floor separation method.
Extraction yield was obtained by the formula
×
The Table summarizes the yield of each Fraction. according to the extracted
Boswellia Serrata, confirmed that the content of the Hexane Fr. was higher than other
Fractions.

66
Figure 12. floor separation diagram of
Boswellia Serrata
.

67
Table 7. The yield of each Fractions according to the extracted Boswellia Serrata

68
4.2. Antibacterial activity using well diffusion method
The results of the antibacterial investigations using the well diffusion method are giv-
en in Table 5. It indicates that different bacterial species demonstrated different levels
of sensitivities towards the tested samples of
B
.
Serrata
extract. The zone of inhibition
of the
B
.
Serrata
extract in all strains was Antibacterial activity for the corresponding
concentrations. The diameter for zone of inhibition for
B
.
Serrata
extract for
S
.
epi-
dermidis
ranged from 13.3±0.58 to 10.3±0.58 mm,
M
.
furfur
ranged from 10.6±0.58
to 9±0 mm,
M
.
pachydermatis
ranged from 13.25±0.35 to 9.75±1.06 mm, and the
Anaerobic jar
C
.
acnes
ranged from 11.83±0.29 to 9.5±0 mm.
In agar well method, the zone of inhibition was larger for all bacteria found to be
sensitive than the disk diffusion method.

69
Table 8. Antibacterial activity of B. Serrata using the disk diffusion method

70
Figure 13. Antibacterial activity of Boswellia Serrata of different concentrations against
M.pachydermatis, M.furfur, S.epidermidis, and C.acnes using the disk diffusion method.

71
4.3. Results of antioxidant activity
4.3.1. DPPH free radical scavenging
Measuring the radical scavenging activity of DPPH is a widely used experimental
method because the antioxidant activity of natural products can easily be measured with
DPPH, a relatively stable free radical, and is closely related to the actual antioxidant
activity. In this experiment, extracts of Boswellia Serrata were prepared at a concen-
tration for each Fraction 10 ⅿg/mL – 100 ⅿg/mL 70% EtOH Fr., 5 ⅿg/mL- 30 ⅿ
g/mL EtOAC Fr., 5 ⅿg/mL – 25 ⅿg/mL Water Fr. to confirm the radical scavenging
capacity of DPPH and measured IC50 values. The results are shown in Table 9, As a
result of the measurement for each Fractions, it was confirmed that there was higher
activity in the Water Fr. compared to the Other fractions, respectively, the Water Fr.
showed the highest scavenging activity 902.19 ± 35.53 µg/mL, Ethyl acetate Fr. 20436
± 652.19 µg/mL ,70% EtOH Fr. 8627.74± 369.22 µg/mL, and the Hexane Fr. Shows
No scavenging activity.

72
Figure 14. DPPH free radical scavenging activity results of Boswellia Serrata extract

73
4.3.2. ABTS radical scavenging
The root scavenging capacity of ABTS was confirmed by preparing Boswellia Serrata
extract at a concentration for each Fraction 5 ⅿg/mL – 30 ⅿg/mL 70% EtOH Fr., 5
ⅿg/mL- 30 ⅿg/mL EtOAC Fr., 10 ⅿg/mL – 75 ⅿg/mL Water Fr. and IC50 values
were measured. The results are shown in Table 9 As a result of the respectively,
which indicated the highest scavenging activity in the Water Fr. it showed a scavenging
activity 1845.08 ± 2265.74 µg/mL, Ethyl acetate Fr. 12167.16 ± 8152.82 µg/mL, 70%
ethanol Fr.13796.44 ± 660.7 µg/mL, the Hexane Fr. Shows No scavenging activity.

74
Figure 15. ABTS radical scavenging activity results of Boswellia Serrata extract.

75
4.4. Results of total polyphenol and flavonoid contents
4.4.1 Total polyphenol contents (TPC)
Polyphenol compounds are one of the secondary metabolites widely distributed in the
plant kingdom and have different structures and molecular weights. Moreover, they bind
easily to proteins and other large molecules due to the phenol hydroxyl group (OH)
and are usually used as antioxidants and anticancer agents. Therefore, in this study, the
total polyphenol content of Boswellia Serrata extract was measured, and the results are
shown in Table 9. As a result of the measurement for each Fractions, it confirmed that
the polyphenol content in the Water Fr. was the highest, it reached 32.15 ± 0.75 ⅿ
g/mL, Ethyl acetate Fr. 9.05 ± 0.91 ⅿg/mL, 70% ethanol Fr. 6.54 ± 1.04 ⅿg/mL, and
Hexane Fr. reached 5.36 ± 0.16 ⅿg/mL.

76
4.4.2. Total flavonoid contents (TFC)
Flavonoids are polyphenol compounds, usually including catechin, quercetin, and rutin,
the most potent antioxidants. It is found in different plants such as stems, leaves, roots,
flowers, and fruits. In this study, the total flavonoid content of Boswellia Serrata ex-
tracts was measured, and the results are shown in Table 9. It also revealed vast differ-
ences in flavonoid contents, with the highest content of flavonoids in Water Fr. 20.29
± 1.47 ⅿg/mL, Ethyl acetate Fr. 8.81 ± 0.14 ⅿg/mL, 70% ethanol Fr. 6.72 ± 0.71 ⅿ
g/mL, and Hexane Fr. 3.12 ± 0.14 ⅿg/mL.

77
Table 9. Radical scavenging activity and polyphenolic/Flavonoids content of
different Boswellia fractions.
IC
50
= The concentration of compound that affords a 50% reduction in the assay; GAE = gallic
acid equivalent. QUE = Quercetine equivalent. - = not detectable.

78
4.5. Analysis results of polyphenol componds
4.5.1 HPLC Analysis Results
HPLC analysis of Boswellia serrata extracts showed that only some of the 13 stand-
ards were detected. The water extract was found to have the highest content of gallic
acid, while the 70% EtOH extract was found to have the highest content of quercetin.
The EtOAc extract was found to have the highest content of epicatechin.

79
No. Sample water 70% EtOH EtOAc
1 Gallic acid 0.19 0.00 0.00
2 Catechin 0.00 0.00 0.20
3 (-)epicatechin 0.00 0.70 1.01
4 vanillic acid 0.00 0.08 0.18
5 Narigin 0.00 0.14 0.23
6 Ethyl gallate 0.00 0.00 0.00
7 p-coumaric acid 0.10 0.07 0.16
8 Ferulic acid 0.00 0.00 0.00
9 Benzoic acid 0.08 0.24 0.64
10 Quercetin 0.01 2.24 0.15
11 Narigenin 0.01 0.04 0.14
12 Kaempferol 0.00 0.00 0.27
13
4-hydroxybenzoic
acid
0.00 0.11 0.17
Total 0.38 3.63 3.16
Table 10. Polyphenol compounds identified in Boswellia serrata extracs quantified by
HPLC (Unit : μg/mg)

80
1
23
4
5
6
7
8
9
10
11
12
13
(D)
R.Time (min)
0 10 20 30 40
2
3
4
5
7
9
10
11
13
(C)
4
3
4
5
7
9
10
11
13
(B)
1
7
9
10
11
13
(A)
R.Time (min)
0 10 20 30 40
Figure 16. HPLC profile of Boswellia serrata extracts and standard mixture using diode
array detection at 280 nm. (A) Boswellia serrata water extract; (B) Boswellia serrata
70% EtOH extract; (C) Boswellia serrata EtOAc extract; (D) standard mixture. Numbers
indicate the following: (1) gallic acid; (2) catechin; (3) (-)-epicatechin; (4) vanillic acid;
(5)Narigin; (6)Ethyl gallate; (7) p-coumaric; (8) ferulic acid; (9) benzoic acid; (10)
quercetin; (11) narigenin; (12) kaempferol; (13) 4-hydroxybenzoic acid

81
4.5.2 HPLC-MS/MS Analysis Results
A total of 14 polyphenol components were identified by HPLC-MS/MS analysis. The
highest content of benzoic acid was found in the 70% EtOH and EtOAc extracts. The
highest content of 5-hydroxymethylfurfural was found in the water extract.

82
No. Compound
Calculated Concentration (μg/g)
Water 70% EtOH EtOAc
1
4-hydroxy benzoic
acid
1.87 0.77 0.64
2 Caffeic acid 0.11 - -
3 Coumaric acid 0.39 - 0.02
4 Naringein - 0.01 0.01
5 Benzoic acid 5.45 13.35 6.82
6 Nicotinic acid 0.07 0.06 0.07
7 Gallic acid - 1.87 1.86
8 Protocatechuic acid 0.26 0.42 0.39
9 Tannic acid - 0.01 0.01
10 Ethyl gallate - 0.02 0.02
11 L-Asparagine 0.18 - -
12
5-hydrox-
ymethylfurfural
6.51 3.62 1.92
13 Chlorogenic acid - 0.40 0.09
14 Quercetin - 0.06 0.05
Total 14.84 20.59 11.9
Table 11. Polyphenol compounds identified in Boswellia serrata extract quantified by
HPLC-MS/MS

83
Figure 17. Component analysis of Boswellia serrata water extract by HPLC MS/MS.

84
Figure 18. Component analysis of Boswellia serrata 70% EtOH extract by HPLC
MS/MS.

85
Figure 19. Component analysis of Boswellia serrata EtOAc extract by HPLC MS/MS.

86
Ⅴ. Discussion
This study highlights the chemical composition and biological activities of Boswellia
serrata and its influential role as a cosmetic ingredient. The results of previous studies
revealed its nutritional, pharmaceutical, and cosmetic benefits as it was used over time.
Boswellia Serrata is a natural source of medicines and cosmetics. It has anti-in-
flammatory, antibacterial, antioxidant, and anti-irritant properties. They also work on an-
ti-aging, anti-acne, brightening, and calming the skin. In addition, it reduces the con-
sequences of acne and spots. It also works well to prevent hair loss and maintain the
health of the scalp and hair[19]
Where the results of the study proved that Boswellia serrata possesses effective anti-
bacterial properties, it only shows us the results of Antibacterial activity using well dif-
fusion method
Based on the results shown in Table 5 provides the findings of the well diffusion
method-based antimicrobial research. It means that various bacterial species showed
varying degrees of sensitivity to the extract of B. Serrata samples that were analyzed.
The B. serrata extract inhibited antibacterial activity in all strains for the relevant
concentrations. The diameter of the zone of inhibition for the B. serrata extract for S.
epidermidis ranged from 13.3±0.58 to 10.3±0.58 mm, for M. furfur from10.6±0.58 to
9±0 mm, for M. pachydermatis from13.25±0.35 to 9.75±1.06 mm, and for C. acnes
from 11.83±0.29 to 9.5±0 mm in the anaerobic jar.
Boswellia serrata has also been shown to be effective to Radical scavenging activity
via diffrent antioxidant assays
Because the antioxidant activity of natural products can be easily measured with
DPPH, a relatively stable free radical, measuring the radical scavenging activity of
DPPH is a widely used experimental technique. In order to confirm the radical scav-
enging ability of DPPH and determine the measured IC50 values, extracts of Boswellia
serrata were produced at a concentration for each fraction of 10 ⅿg/mL – 100 ⅿ
g/mL 70% EtOH Fr., 5 ⅿg/mL- 30 ⅿg/mL EtOAC Fr., 5 ⅿg/mL – 25 ⅿg/mL

87
Water Fr.
As a consequence of the measurement for each fraction, the findings are displayed in
Table 9. It was confirmed that there was higher activity in the Water Fr. compared to
the Other fractions; respectively, the Water Fr. showed the highest scavenging activity
902.19 ± 35.53 µg/mL, Ethyl acetate Fr. 20436 ± 652.19 µg/mL ,70% EtOH Fr.
8627.74± 369.22 µg/mL, and the Hexane Fr. Shows No scavenging activity.
By preparing Boswellia Serrata extracts at a concentration for each Fraction, the root
scavenging ability of ABTS was verified. concentration for each Fraction 5 ⅿg/mL –
30 ⅿg/mL 70% EtOH Fr. , 5 ⅿg/mL- 30 ⅿg/mL EtOAC Fr., 10 ⅿg/mL – 75 ⅿ
g/mL Water Fr. IC50 values were measured. Table 9 presents the findings. Accordingly,
the Water fraction demonstrated the maximum scavenging activity with a scavenging ac-
tivity of 1845.08 ± 2265.74 , followed by the ethyl acetate fraction with a scavenging
activity of 12167.16 ± 8152.82 µg/mL and the 70% ethanol fraction with a scavenging
activity of 13796.44 ± 660.7 µg/mLHexane Fr. Shows No scavenging activity.
In the total polyphenol content of Boswellia Serrata extracts was measured, our result
of the measurement for each Fraction confirmed that the polyphenol content in the
Water Fr. was the highest; it reached 32.15 ± 0.75 ⅿg/mL, Ethyl acetate Fr. 9.05 ±
0.91 ⅿg/mL, 70% ethanol Fr. 6.54 ± 1.04 ⅿg/mL, and Hexane Fr. reached 5.36 ±
0.16 ⅿg/mL.
We found that the total flavonoid content of Boswellia Serrata extracts also revealed
vast differences in flavonoid contents, with the highest content of flavonoids in Water
Fr. 20.29 ± 1.47 ⅿg/mL, Ethyl acetate Fr. 8.81 ± 0.14 ⅿg/mL, 70% ethanol Fr. 6.72
± 0.71 ⅿg/mL, and Hexane Fr. 3.12 ± 0.14 ⅿg/mL.
HPLC analysis uses standards to perform qualitative analysis, while LC-MS/MS anal-
ysis performs both qualitative and quantitative analysis. Since HPLC analyzes by com-
paring the retention time with a standard, the peak at the same time may contain mul-
tiple substances. LC-MS/MS analysis separates the substances through HPLC, and then
each component is analyzed qualitatively and quantitatively through a mass spectrometer.
Since it is not a simple separation analysis, after HPLC analysis, LC-MS/MS analysis

88
is performed for accurate analysis. Therefore, the results of HPLC analysis and
LC-MS/MS analysis are bound to be different. In this study, HPLC analysis confirmed
that the highest content of gallic acid in the water extract, quercetin in the 70% EtOH
extract, and epicatechin in the EtOAc extract were found in the water extract.
LC-MSMS analysis confirmed the highest content of 5-hydroxymethylfurfural in the wa-
ter extract and benzoic acid in the 70% EtOH and EtOAc extracts. The reason for the
different analytical results is due to the difference in the analytical methods of the ana-
lyzing instruments. In addition, the unknown samples that were not identified in the
HPLC analysis require further study and substance identification using analytical instru-
ments other than HPLC-MS/MS.

89
Ⅵ. Conculusion
Boswellia serrata resin is an important oleo-gum resin used in many industries, in-
cluding the pharmaceutical, culinary, flavoring, alcoholic beverage, cosmetic, and per-
fume industries.
It is one of aromatherapy's most commonly used essential oils to treat breathing
disorders. It is also used to treat various bacterial and fungal infections.
From the current study, it is concluded that Boswellia serrata resin has significant
antibacterial and antioxidant activities. As it has proven effective in discouraging bacte-
rial strains that cause skin diseases and grains such a
S
.
epidermidis
,
M
.
furfur
,
M
.
pachydermatis
and
C
.
acnes
, and it also has the ability to resist free radicals. This
means it has many uses and applications in cosmetics, including anti-aging, soothing,
and anti-inflammatory properties. It can also encourage faster skin regeneration and in-
crease skin elasticity to lighten the complexion.
Global cosmetic companies have recently relied on manufacturing skin, hair and
body care products from natural plant sources. Which means that finding natural sour-
ces It is easier to get the most out of these plants when they have cosmetic features
and are used in the creation of cosmetics. This also helps to broaden the sources and
is likely to reduce environmental losses, such as burning plants and dumping environ-
mental trash. This increases income and contributes to the economic growth of the
country exporting these plants.
For future Works Expecting more research to analyze polyphenol componds that
were not identified in the HPLC analysis require further study and substance identi-
fication using analytical instruments other than HPLC-MS/MS.

90
Ⅶ. Reference
1. Larsson, S. C., Bergkvist, L., Näslund, I., Rutegård, J., & Wolk, A. (2007).
Vitamin A, retinol, and carotenoids and the risk of gastric cancer: a prospective cohort
study. The American journal of clinical nutrition, 85(2), 497-503.
2. Ko, R. J. (1998). Adulterants in Asian patent medicines. New England Journal of
Medicine, 339(12), 847-847.
3. Glaser, D. A. (2004). Anti-aging products and cosmeceuticals. Facial Plastic
Surgery Clinics, 12(3), 363-372.
4. Dalirsani, Z., & Adibpour, M. (2011). In vitro comparision of inhibiyory activity
of 10 plant extracts against Candida albicans 930-935
5. Gibbons, S. (2008). Phytochemicals for bacterial resistance-strengths, weaknesses
and opportunities. Planta medica, 74(06), 594-602.
6. Rousseaux, C. G., & Schachter, H. (2003). Regulatory issues concerning the safe-
ty, efficacy and quality of herbal remedies. Birth Defects Research Part B:
Developmental and Reproductive Toxicology, 68(6), 505-510.
7. Kole, P. L., Jadhav, H. R., Thakurdesai, P., & Nagappa, A. N. (2005). Cosmetic
potential of herbal extracts.
8. Shivanand, P., Nilam, M., & Viral, D. (2010). Herbs play an important role in the
field of cosmetics. International Journal of PharmTech Research, 2(1), 632-639.
9. Khan, M. A., Ali, R., Parveen, R., Najmi, A. K., & Ahmad, S. (2016).
Pharmacological evidences for cytotoxic and antitumor properties of Boswellic acids
from Boswellia serrata. Journal of ethnopharmacology, 191, 315-323.
10. Howes, F. N. (1950). Age-old resins of the Mediterranean region and their uses.
Economic Botany, 4(4), 307-316.
11. Holmes, P. (1999). Frankincense oil. Int J Arom, 9, 56-61.
12. Rüdiger, A. L., Siani, A. C., & Junior, V. V. (2007). The chemistry and pharma-
cology of the South America genus Protium Burm. f.(Burseraceae). Pharmacognosy re-

91
views, 1(1), 93-104.
13. Hillson, R. M. (1988). Gold, frankincense and myrrh. Journal of the Royal
Society of Medicine, 81(9), 542-543.
14. Al‐Saidi, S., Rameshkumar, K. B., Hisham, A., Sivakumar, N., & Al‐Kindy, S.
(2012). Composition and Antibacterial Activity of the Essential Oils of Four
Commercial Grades of Omani Luban, the Oleo‐Gum Resin of Boswellia sacra Flueck.
Chemistry & Biodiversity, 9(3), 615-624.
15. Weckesser, S., Engel, K., Simon-Haarhaus, B., Wittmer, A., Pelz, K., &
Schempp, C. Á. (2007). Screening of plant extracts for antimicrobial activity against
bacteria and yeasts with dermatological relevance. Phytomedicine, 14(7-8), 508-516.
16. Aman, M., Ravishankar Rai, V., & Samaga, P. V. (2010). Antimicrobial and
Phytochemical Screening of Boswellia serrata Roxb., Rhus mysorensis Heyne, Strychnos
potatorum Linn. F. and Schefflera stellata Gaertn. Medicinal and Aromatic Plant Science
and Biotechnology, 4(1), 69-72.
17. Sultan, F. I. (2020). Phytochemical analysis and antibacterial activities of
Frankincense of Boswellia serrate. Plant Archives, 20(2), 5219-5226.
18. Banno, N., Akihisa, T., Yasukawa, K., Tokuda, H., Tabata, K., Nakamura, Y., ...
& Suzuki, T. (2006). Anti-inflammatory activities of the triterpene acids from the resin
of Boswellia carteri. Journal of ethnopharmacology, 107(2), 249-253.
19. Alraddadi, B. G., & Shin, H. J. (2022). Biochemical Properties and Cosmetic
Uses of Commiphora myrrha and Boswellia serrata. Cosmetics, 9(6), 119.
20. Soni, A., & Bohra, N. K. (2021). Boswelliaserrata-Propogation and uses-A
Review. Int. J. Adv. Res. Biol., Sci, 8(5), 35-46.
21. Sharma, A., Mann, A., Gajbhiye, V., & Kharya, M. (2007). PHCOG REV.: plant
review phytochemical profile of Boswellia serrata: an overview. Pharmacogn Rev, 1(1),
131-142.
22. Siddiqui, M. Z. (2011). Boswellia serrata, a potential antiinflammatory agent: an
overview. Indian journal of pharmaceutical sciences, 73(3), 255.

92
23. Ismail, S. M., Aluru, S., Sambasivarao, K. R. S., & Matcha, B. (2014).
Antimicrobial activity of frankincense of Boswellia serrata. Int. J. Curr. Microbiol. App.
Sci, 3(10), 1095-1101.
24. Salman, K. A., Jawad, S. M., & Abbas, S. H. (2021). Evaluation of antibacterial
activity of Boswellia Serrata extract against some of the oral pathogenic bacteria. Indian
Journal of Forensic Medicine & Toxicology, 15(3), 3371-3376.
25. Alam, M., Khan, H., Samiullah, L., & Siddique, K. M. (2012). A review on
phytochemical and pharmacological studies of Kundur (Boswellia serrata Roxb ex
Colebr.)-A Unani drug. Journal of Applied Pharmaceutical Science Issue, 148-156.
26. Zutshi, U., Rao, P. G., Kaur, S., & Atal, G. S. C. (1980). Mechanism of choles-
terol lowering effect of Salai guggal ex-Boswellia serrata. Indian J Pharm, 12, 59.
27. Ahmed, H. H., El-Alfy, N. Z., Mahmoud, M. F., & Yahya, S. M. (2015).
Boswellia serrata oleo-gum resin: a natural remedy for retrogradation of liver fibrosis in
rats. Der Pharmacia Lettre, 7(1), 134-144.
28. Yassin, N., El-Shenawy, S., Mahdy, K. A., Gouda, N., Marrie, A. E. F. H.,
Farrag, A., & Ibrahim, B. M. (2013). Effect of Boswellia serrata on Alzheimer’s dis-
ease induced in rats. J Arab Soc Med Res, 8, 1-11.
29. Kumar, R., Singh, S., Saksena, A. K., Pal, R., Jaiswal, R., & Kumar, R. (2019).
Effect of Boswellia serrata extract on acute inflammatory parameters and tumor necrosis
factor-α in complete Freund's adjuvant-induced animal model of rheumatoid arthritis.
International Journal of Applied and Basic Medical Research, 9(2), 100.
30. Sengupta, K., Krishnaraju, A. V., Vishal, A. A., Mishra, A., Trimurtulu, G.,
Sarma, K. V., ... & Raychaudhuri, S. P. (2010). Comparative efficacy and tolerability
of 5-Loxin® and Aflapin® against osteoarthritis of the knee: a double blind, random-
ized, placebo controlled clinical study. International journal of medical sciences, 7(6),
366.
31. Ali, N. A. A., Wurster, M., Arnold, N., Teichert, A., Schmidt, J., Lindequist, U.,
& Wessjohann, L. (2008). Chemical composition and biological activities of essential

93
oils from the oleogum resins of three endemic Soqotraen Boswellia species. Rec. Nat.
Prod, 2(6).
32. Majeed, M., Majeed, S., Narayanan, N. K., & Nagabhushanam, K. (2019). A pi-
lot, randomized, double‐blind, placebo‐controlled trial to assess the safety and efficacy
of a novel Boswellia serrata extract in the management of osteoarthritis of the knee.
Phytotherapy Research, 33(5), 1457-1468.
33. Jaroš, P., Vrublevskaya, M., Lokočová, K., Michailidu, J., Kolouchová, I., &
Demnerová, K. (2022). Boswellia serrata Extract as an Antibiofilm Agent against
Candida spp. Microorganisms, 10(1), 171.
34. Ayub, M. A., Hanif, M. A., Sarfraz, R. A., & Shahid, M. (2018). Biological ac-
tivity of Boswellia serrata Roxb. oleo gum resin essential oil: effects of extraction by
supercritical carbon dioxide and traditional methods. International Journal of Food
Properties, 21(1), 808-820.
35. Lemenith, M., & Teketay, D. (2003). Frankincense and myrrh resources of
Ethiopia: II. Medicinal and industrial uses. SINET: Ethiopian Journal of Science, 26(2),
161-172.
36. Tucker, A. O. (1986). Frankincense and myrrh. Economic botany, 40(4), 425-433.
37. Feng, P. M., Lin, H., & Chen, W. (2013). Identification of antioxidants from se-
quence information using naive Bayes. Computational and mathematical methods in
medicine, 2013.
38. Sies, H. (1997). Oxidative stress: oxidants and antioxidants. Experimental
Physiology: Translation and Integration, 82(2), 291-295.
39. Katiyar, S. K., & Mukhtar, H. (2001). Green tea polyphenol (−)‐epigallocatechin‐
3‐gallate treatment to mouse skin prevents UVB‐induced infiltration of leukocytes, deple-
tion of antigen‐presenting cells, and oxidative stress. Journal of Leukocyte Biology,
69(5), 719-726.
40. Sander, C. S., Chang, H., Salzmann, S., Müller, C. S., Ekanayake-Mudiyanselage,
S., Elsner, P., & Thiele, J. J. (2002). Photoaging is associated with protein oxidation in

94
human skin in vivo. Journal of Investigative Dermatology, 118(4), 618-625.
41. Poljšak, B., & Dahmane, R. (2012). Free radicals and extrinsic skin aging.
Dermatology research and practice, 2012.
42. Hanson, K. M., & Clegg, R. M. (2002). Observation and Quantification of
Ultraviolet‐induced Reactive Oxygen Species in Ex Vivo Human Skin¶. Photochemistry
and photobiology, 76(1), 57-63.
43. Pattison, D. I., & Davies, M. J. (2006). Actions of ultraviolet light on cellular
structures. Cancer: cell structures, carcinogens and genomic instability, 131-157.
44. Shindo, Y., Witt, E., & Packer, L. (1993). Antioxidant defense mechanisms in
murine epidermis and dermis and their responses to ultraviolet light. Journal of inves-
tigative dermatology, 100(3), 260-265.
45. Shindo, Y., Witt, E., Han, D., Tzeng, B., Aziz, T., Nguyen, L., & Packer, L.
(1994). Recovery of antioxidants and reduction in lipid hydroperoxides in murine epi-
dermis and dermis after acute ultraviolet radiation exposure. Photodermatology, photo-
immunology & photomedicine, 10(5), 183-191.
46. Thiele, J., Barland, C. O., Ghadially, R., & Elias, P. M. (2006). Permeability and
antioxidant barriers in aged epidermis. Skin aging, 65-79.
47. Weber, S. U., Thiele, J. J., Packer, L., & Cross, C. E. (1999). Vitamin C, uric
acid, and glutathione gradients in murine stratum corneum and their susceptibility to
ozone exposure. Journal of Investigative Dermatology, 113(6), 1128-1132.
48. Harder, J., Bartels, J., Christophers, E., & Schröder, J. M. (1997). A peptide anti-
biotic from human skin. Nature, 387(6636), 861-861.
49. Chiller, K., Selkin, B. A., & Murakawa, G. J. (2001). Skin microflora and bacte-
rial infections of the skin. In Journal of Investigative dermatology Symposium proceed-
ings (Vol. 6, No. 3, pp. 170-174). Elsevier.
50. Leyden, J. J. (1991). Cutaneous microbiology. Physiology, Biochemistry and
Molecular biology of the Skin, 1403-1424.
51. Feingold, D. S. (1986). Bacterial adherence, colonization, and pathogenicity.

95
Archives of Dermatology, 122(2), 161-163.
52. Bibel, D. J., Aly, R., Bayles, C., Strauss, W. G., Shinefield, H. R., & Maibach,
H. I. (1983). Competitive adherence as a mechanism of bacterial interference. Canadian
journal of microbiology, 29(6), 700-703.
53. Peterson, P. K., Verhoef, J., Sabath, L. D., & Quie, P. G. (1976). Extracellular
and bacterial factors influencing staphylococcal phagocytosis and killing by human poly-
morphonuclear leukocytes. Infection and immunity, 14(2), 496-501.
54. Hentges, D. J. (1993). The anaerobic microflora of the human body. Clinical in-
fectious diseases, 16(Supplement_4), S175-S180.
55. Iwase, T., Uehara, Y., Shinji, H., Tajima, A., Seo, H., Takada, K., ... &
Mizunoe, Y. (2010). Staphylococcus epidermidis Esp inhibits Staphylococcus aureus bio-
film formation and nasal colonization. Nature, 465(7296), 346-349.
56. Hedrick, J. (2003). Acute bacterial skin infections in pediatric medicine: current
issues in presentation and treatment. Paediatric Drugs, 5, 35-46.
57. Ghannoum, M. A., Jurevic, R. J., Mukherjee, P. K., Cui, F., Sikaroodi, M.,
Naqvi, A., & Gillevet, P. M. (2010). Characterization of the oral fungal microbiome
(mycobiome) in healthy individuals. PLoS pathogens, 6(1), e1000713.
58. Fierer, N., Lauber, C. L., Zhou, N., McDonald, D., Costello, E. K., & Knight,
R. (2010). Forensic identification using skin bacterial communities. Proceedings of the
National Academy of Sciences, 107(14), 6477-6481.
59. Leyden, J. J., Stewart, R., & Kligman, A. M. (1980). Experimental infections
with group A streptococci in humans. Journal of Investigative Dermatology, 75(2),
196-201.
60. Dillon, H. C. (1968). Impetigo contagiosa: suppurative and non-suppurative com-
plications: I. Clinical, bacteriologic, and epidemiologic characteristics of impetigo.
American Journal of Diseases of Children, 115(5), 530-541.
61. Dillon Jr, H. C., & Reeves, M. S. A. (1974). Streptococcal immune responses in
nephritis after skin infection. The American Journal of Medicine, 56(3), 333-346.

96
62. Strauss, J., & Strauss, L. (Eds.). (2012). Acute Renal Disorders and Renal
Emergencies: Proceedings of Pediatric Nephrology Seminar X Held at Bal Harbour,
Florida, January 30–February 3, 1983 (Vol. 7). Springer Science & Business Media.
63. Celestin, R., Brown, J., Kihiczak, G., & Schwartz, R. A. (2007). Erysipelas: a
common potentially dangerous infection. ACTA DERMATOVENEROLOGICA ALPINA
PANONICA ET ADRIATICA, 16(3), 123.
64. Heng, M. C. Y., Khoo, M., Cooperman, A., & FALLON‐FRIEDLANDER, S.
(1994). Haemorrhagic cellulitis: a syndrome associated with tumour necrosis factor‐α.
British Journal of Dermatology, 130(1), 65-74.
65. Von Nussbaum, F., Brands, M., Hinzen, B., Weigand, S., & Häbich, D. (2006).
Antibacterial natural products in medicinal chemistry—exodus or revival?. Angewandte
Chemie International Edition, 45(31), 5072-5129.
66. Witte, W. (2004). International dissemination of antibiotic resistant strains of bac-
terial pathogens. Infection, Genetics and Evolution, 4(3), 187-191.
67. Kumar, A., Kumar, S., Ramchiary, N., & Singh, P. (2021). Role of traditional
ethnobotanical knowledge and indigenous communities in achieving Sustainable
Development Goals. Sustainability, 13(6), 3062.
68. Atanasov, A. G., Waltenberger, B., Pferschy-Wenzig, E. M., Linder, T.,
Wawrosch, C., Uhrin, P., ... & Stuppner, H. (2015). Discovery and resupply of pharma-
cologically active plant-derived natural products: A review. Biotechnology advances,
33(8), 1582-1614.
69. Sofowora, A., Ogunbodede, E., & Onayade, A. (2013). The role and place of
medicinal plants in the strategies for disease prevention. African journal of traditional,
complementary and alternative medicines, 10(5), 210-229.
70. Elsner, P., & Maibach, H. I. (Eds.). (2000). Cosmeceuticals: drugs vs. cosmetics
(Vol. 23). CRC Press.
71. Amberg, N., & Fogarassy, C. (2019). Green consumer behavior in the cosmetics
market. Resources, 8(3), 137.

97
72. Esty, D. C., & Simmons, P. J. (2011). The green to gold business playbook:
How to implement sustainability practices for bottom-line results in every business
function. John Wiley & Sons.
73. Fowler, G. J., & Malandkar, M. A. (1921). A Suggested Method for the
Extraction of Turpentine, Resin and Gum from the Gum-oleo-resin of Boswellia Serrata
without the use of Solvents. Journal of the Indian Institute of Science, 4, 27-42.
74. Malandkar, M. A. (1925). II. Chemical Constitution of the Gum from Boswellia
serrata. Journal of the Indian Institute of Science, 8(Part A), 221-244.
75. Togni, S., Maramaldi, G., Di Pierro, F., & Biondi, M. (2014). A cosmeceutical
formulation based on boswellic acids for the treatment of erythematous eczema and
psoriasis. Clinical, cosmetic and investigational dermatology, 321-327.
76. Katragunta, K., Siva, B., Kondepudi, N., Vadaparthi, P. R., Rao, N. R., Tiwari,
A. K., & Babu, K. S. (2019). Estimation of boswellic acids in herbal formulations con-
taining Boswellia serrata extract and comprehensive characterization of secondary metab-
olites using UPLC-Q-Tof-MSe. Journal of Pharmaceutical Analysis, 9(6), 414-422.
77. Poeckel, D., & Werz, O. (2006). Boswellic acids: biological actions and molec-
ular targets. Current medicinal chemistry, 13(28), 3359-3369.
78. Ammon, H. P. T. (2006). Boswellic acids in chronic inflammatory diseases.
Planta medica, 72(12), 1100-1116.
79. Ahmed, H. H., Abd-Rabou, A. A., Hassan, A. Z., & Kotob, S. E. (2015).
Phytochemical analysis and anti-cancer investigation of Boswellia serrata bioactive con-
stituents in vitro. Asian Pacific Journal of Cancer Prevention, 16(16), 7179-7188.
80. Yuan, G., Wahlqvist, M. L., He, G., Yang, M., & Li, D. (2006). Natural prod-
ucts and anti-inflammatory activity. Asia Pacific journal of clinical nutrition, 15(2).
81. Kokate, C. K., Purohit, A. P., & Gokhale, S. B. (1999). Resin and resin
combinations. Pharmacognosy Nirali Prakashan, Pune.
82. Culioli, G., Mathe, C., Archier, P., & Vieillescazes, C. (2003). A lupane tri-
terpene from frankincense (Boswellia sp., Burseraceae). Phytochemistry, 62(4), 537-541.

98
83. Kumar, A., & Saxena, V. K. (1979). TLC and GLC studies of the essential oil
from Boswellia serrata leaves. Indian drugs.
84. Ganzera, M., Stöggl, W. M., Bonn, G. K., Khan, I. A., & Stuppner, H. (2003).
Capillary electrochromatography of boswellic acids in Boswellia serrata Roxb. Journal of
separation science, 26(15‐16), 1383-1388.
85. Wahab, S. A., Aboutabl, E. A., El-Zalabani, S. M., Fouad, H. A., De Pooter, H.
L., & El-Fallaha, B. (1987). The essential oil of olibanum. Planta medica, 53(04),
382-384.
86. Rastogi, R. P., & Mehrotra, B. N. (1990). Compendium of Indian medicinal
plants. Central Drug Research Institute.
87. Al-Harrasi, A., & Al-Saidi, S. (2008). Phytochemical analysis of the essential oil
from botanically certified oleogum resin of Boswellia sacra (Omani Luban). Molecules,
13(9), 2181-2189.
88. Rashan, L., Hakkim, F. L., Idrees, M., Essa, M., Velusamy, T., Al-Baloshi, M.,
... & Hasson, S. S. (2019). Boswellia gum resin and essential oils: potential health ben-
efits− an evidence based review. International Journal of Nutrition, Pharmacology,
Neurological Diseases, 9(2), 53-71.
89. Al-Yasiry, A. R. M., & Kiczorowska, B. (2016). Frankincense–therapeutic
properties. Advances in Hygiene and Experimental Medicine, 70, 380-391.
90. Niebler, J., & Buettner, A. (2015). Identification of odorants in frankincense
(Boswellia sacra Flueck.) by aroma extract dilution analysis and two-dimensional gas
chromatography–mass spectrometry/olfactometry. Phytochemistry, 109, 66-75.
91. Al-Harrasi, A., Csuk, R., Khan, A., & Hussain, J. (2019). Distribution of the an-
ti-inflammatory and anti-depressant compounds: Incensole and incensole acetate in genus
Boswellia. Phytochemistry, 161, 28-40.
92. Gangwal, M. L., & Vardhan, D. K. (1995). Carbohydrate contents of Boswellia
serrata. Asian Journal of Chemistry, 7(3), 677.
93. Faccio, G. (2020). Plant complexity and cosmetic innovation. IScience, 23(8),

99
101358.
94. Ahshawat, M. S., Saraf, S., & Saraf, S. (2008). Preparation and characterization
of herbal creams for improvement of skin viscoelastic properties. International journal of
cosmetic science, 30(3), 183-193.
95. Farage, M. A., Miller, K. W., Elsner, P., & Maibach, H. I. (2007). Structural
characteristics of the aging skin: a review. Cutaneous and ocular toxicology, 26(4),
343-357.
96. Tortora, G. J., Funke, B. R., & Case, C. L. (2018). Microbiology: an
introduction. Pearson.
97. de Lima Cherubim, D. J., Buzanello Martins, C. V., Oliveira Fariña, L., & da
Silva de Lucca, R. A. (2020). Polyphenols as natural antioxidants in cosmetics
applications. Journal of cosmetic dermatology, 19(1), 33-37.
98. Younas, A., Naqvi, S. A., Khan, M. R., Shabbir, M. A., Jatoi, M. A., Anwar,
F., ... & Aadil, R. M. (2020). Functional food and nutra‐pharmaceutical perspectives of
date (Phoenix dactylifera L.) fruit. Journal of food biochemistry, 44(9), e13332.
99. Calabrese, V., Scapagnini, G., Catalano, C., Dinotta, F., Geraci, D., & Morganti,
P. (2000). Biochemical studies of a natural antioxidant isolated from rosemary and its
application in cosmetic dermatology. International journal of tissue reactions, 22(1),
5-13.
100. Hourfane, S., Mechqoq, H., Errajouani, F., Rocha, J. M., & El Aouad, N.
(2022). In Vitro and In Silico Evaluations of Boswellia carterii Resin Dermocosmetic
Activities. Cosmetics, 9(6), 131.
101. Yasmeen, S., & Gupta, P. (2019). Interaction of Selected Terpenoids From With
Catalytic Domain of Matrix Metalloproteinase-1: An In Silico Assessment of Their
Anti-wrinkling Potential. Bioinformatics and Biology Insights.
102. Hamidpour, R., Hamidpour, S., Hamidpour, M., & Shahlari, M. (2013).
Frankincense (Boswellia Species): From the selection of traditional applications to the
novel phytotherapy for the prevention and treatment of serious diseases. Journal of tra-

100
ditional and complementary medicine, 3(4), 221-226.
103. Eyre, H., Hills, M. J., & Watkins, S. D. (2003). U.S. Patent No. 6,589,516.
Washington, DC: U.S. Patent and Trademark Office.
104. Saafi, E. B., Trigui, M., Thabet, R., Hammami, M., & Achour, L. (2008).
Common date palm in Tunisia: chemical composition of pulp and pits. International
journal of food science & technology, 43(11), 2033-2037.
105. Hong, Y. J., Tomas-Barberan, F. A., Kader, A. A., & Mitchell, A. E. (2006).
The flavonoid glycosides and procyanidin composition of Deglet Noor dates (Phoenix
dactylifera). Journal of agricultural and food chemistry, 54(6), 2405-2411.
106. Adhirajan, N., Kumar, T. R., Shanmugasundaram, N., & Babu, M. (2003). In
vivo and in vitro evaluation of hair growth potential of Hibiscus rosa-sinensis Linn.
Journal of ethnopharmacology, 88(2-3), 235-239.
107. Aris, N., Norhuda, I., & Adeib, I. (2013). Extraction of Phoenix dactylifera
(Mariami) seeds oil using supercritical carbon dioxide (SC-CO2). International Journal,
4(1).
108. Kostik, V., Memeti, S., & Bauer, B. (2013). Fatty acid composition of edible
oils and fats. Journal of Hygienic Engineering and Design, 4, 112-116.
109. Kwon, O. S., Han, J. H., Yoo, H. G., Chung, J. H., Cho, K. H., Eun, H. C.,
& Kim, K. H. (2007). Human hair growth enhancement in vitro by green tea epi-
gallocatechin-3-gallate (EGCG). Phytomedicine, 14(7-8), 551-555.
110. Cheynier, V. (2012). Phenolic compounds: from plants to foods. Phytochemistry
reviews, 11(2-3), 153-177.
111. Majeed, M., Majeed, S., Nagabhushanam, K., Mundkur, L., Neupane, P., &
Shah, K. (2020). Clinical study to evaluate the efficacy and safety of a hair serum
product in healthy adult male and female volunteers with hair fall. Clinical, cosmetic
and investigational dermatology, 691-700.
112. Al-Alawi, R. A., Al-Mashiqri, J. H., Al-Nadabi, J. S., Al-Shihi, B. I., & Baqi,
Y. (2017). Date palm tree (Phoenix dactylifera L.): natural products and therapeutic

101
options. Frontiers in plant science, 8, 845.
113. Habib, H. M., Platat, C., Meudec, E., Cheynier, V., & Ibrahim, W. H. (2014).
Polyphenolic compounds in date fruit seed (Phoenix dactylifera): characterisation and
quantification by using UPLC‐DAD‐ESI‐MS. Journal of the Science of Food and
Agriculture, 94(6), 1084-1089.
114. Haider, M. S., Khan, I. A., Naqvi, S. A., Jaskani, M. J., Khan, R. W., Nafees,
M., & Pasha, I. (2013). Fruit developmental stages effects on biochemical attributes in
date palm. Pakistan Journal of Agricultural Sciences, 50(4).
115. Walke, D. D., & Daud, F. S. (2018). Date palm fruit (Phoenix dactylifera L.)
as a cosmetic ingredient. JETIR, 5, 755-762.
116. Dattola, A., Silvestri, M., Bennardo, L., Passante, M., Scali, E., Patruno, C., &
Nisticò, S. P. (2020). Role of vitamins in skin health: A systematic review. Current nu-
trition reports, 9, 226-235.
117. Mansouri, A., Embarek, G., Kokkalou, E., & Kefalas, P. (2005). Phenolic profile
and antioxidant activity of the Algerian ripe date palm fruit (Phoenix dactylifera). Food
chemistry, 89(3), 411-420.
118. Kchaou, W., Abbès, F., Blecker, C., Attia, H., & Besbes, S. (2013). Effects of
extraction solvents on phenolic contents and antioxidant activities of Tunisian date vari-
eties (Phoenix dactylifera L.). Industrial crops and products, 45, 262-269.
119. DiBaise, M., & Tarleton, S. M. (2019). Hair, nails, and skin: differentiating cu-
taneous manifestations of micronutrient deficiency. Nutrition in Clinical Practice, 34(4),
490-503.
120. BOUDAYA, S., KESKES, L., BESBES, S., EL GAIED, A., ATTIA, H.,
TURKI, H., & HENTATI, B. (2007). Effects of date seed oil on normal human skin in
vitro. European Journal of Dermatology, 17(6), 516-9.
121. Al-Farsi, M. A., & Lee, C. Y. (2008). Optimization of phenolics and dietary fi-
bre extraction from date seeds. Food chemistry, 108(3), 977-985.
122. Fernández, E., Martínez-Teipel, B., Armengol, R., Barba, C., & Coderch, L.

102
(2012). Efficacy of antioxidants in human hair. Journal of Photochemistry and
Photobiology B: Biology, 117, 146-156.
123. Baliga, M. S., Baliga, B. R. V., Kandathil, S. M., Bhat, H. P., & Vayalil, P.
K. (2011). A review of the chemistry and pharmacology of the date fruits (Phoenix
dactylifera L.). Food research international, 44(7), 1812-1822.
124. Saleh, E. A., Tawfik, M. S., & Abu-Tarboush, H. M. (2011). Phenolic contents
and antioxidant activity of various date palm (Phoenix dactylifera L.) fruits from Saudi
Arabia. Food and Nutrition Sciences, 2011.
125. Gupta, I., Parihar, A., Malhotra, P., Singh, G. B., Lüdtke, R., Safayhi, H., &
Ammon, H. P. (1997). Effects of Boswellia serrata gum resin in patients with ulcerative
colitis. European journal of medical research, 2(1), 37-43.
126. Birkner, K. M. (2006). Boswellia, the pain herb. Pain and Stress Publications,
1(1), 60-1.
127. Raja, A. F., Ali, F., Khan, I. A., Shawl, A. S., & Arora, D. S. (2011).
Acetyl-11-keto-β-boswellic acid (AKBA); targeting oral cavity pathogens. BMC research
notes, 4(1), 1-8.
128. Gomaa, A. A., Makboul, R. M., Al-Mokhtar, M. A., & Nicola, M. A. (2019).
Polyphenol-rich Boswellia serrata gum prevents cognitive impairment and insulin resist-
ance of diabetic rats through inhibition of GSK3β activity, oxidative stress and pro-in-
flammatory cytokines. Biomedicine & Pharmacotherapy, 109, 281-292.
129. Al-Yahya, A. A., Asad, M., Sadaby, A., & Alhussaini, M. S. (2020). Repeat or-
al dose safety study of standardized methanolic extract of Boswellia sacra oleo gum
resin in rats. Saudi Journal of Biological Sciences, 27(1), 117-123.
130. Lalithakumari, K., Krishnaraju, A. V., Sengupta, K., Subbaraju, G. V., &
Chatterjee, A. (2006). Safety and toxicological evaluation of a novel, standardized
3-O-acetyl-11-keto-β-boswellic acid (AKBA)-enriched Boswellia serrata extract
(5-Loxin®). Toxicology Mechanisms and Methods, 16(4), 199-226.
131. Patel, S. S., & Savjani, J. K. (2015). Systematic review of plant steroids as po-

103
tential antiinflammatory agents: Current status and future perspectives. The journal of
phytopharmacology, 4(2), 121-125.
132. Mishra, S. U. D. H. A. N. S. H. U., Bishnoi, R. S., Maurya, R. A. H. U. L.,
& Jain, D. E. E. P. T. I. (2020). Boswellia Serrata ROXB.–a Bioactive Herb with
Various Pharmacological Activities. Asian J. Pharm. Clin. Res, 13(11), 33-39.
133. Chand, B. (2013). Antibacterial effect of garlic (Allium sativum) and ginger
(Zingiber officinale) against Staphylococcus aureus, Salmonella typhi, Escherichia coli
and Bacillus cereus. Journal of microbiology, biotechnology and food sciences, 2(4),
2481-2491.
134. Singh, H. P., Yadav, I. K., Chandra, D., & Jain, D. A. (2012). In vitro anti-
oxidant and free radical scavenging activity of different extracts of Boerhavia diffusa
and Boswellia serrata. International Journal of Pharma Sciences and Research, 3(11),
503-511.
135. Gupta, M., Singh, S., Luqman, S., Saikia, D., Thomas, M., & Rout, P. K.
(2022). Correlation of boswellic acids with antiproliferative, antioxidant and anti-
microbial activities of topographically collected Boswellia serrata oleo-gum-resin.
Phytomedicine Plus, 2(3), 100289.
