
Instructions for Use of 2023 BIT Part F
WORKBOOK
CLSI Version 1.0. This was last updated on 15 May 2023 and has been approved by CLSI’s Outreach Working Group.
This is Part E of the of the 2023 Breakpoint Implementation Toolkit.
To access the entire BIT Toolkit, visit https://clsi.org/bit-toolkit/.

2
Accuracy Instructions for Prelled Excel
Table of Contents
Workbook Overview ............................................................................3
Accuracy .......................................................................................6
Using the Excel for Accuracy .......................................................................6
Clearing Test Data .............................................................................8
Analysis Within the Breakpoint Implementation Toolkit Set Worksheets .........................9
Precision/Reproducibility ......................................................................10
Using the Excel for Precision (Reproducibility) ................................................. 10
Reviewing Aggregated Summary Data ..........................................................11
Unlocking the Spreadsheet and Hidden Columns ................................................13

3
Accuracy Instructions for Prelled Excel
Accuracy Instructions for Prelled Excel
Workbook Overview
1. This workbook contains Microsoft Excel VBA Macros.
2. This workbook can be used to summarize and determine the performance of a test generating
minimum inhibitory concentration (MIC) values (eg, broth microdilution panel, MIC card, or
gradient diffusion strips) using specic CDC and FDA Antimicrobial Resistance Isolate Bank (AR
Bank) Breakpoint Implementation Toolkit (BIT) isolate sets.
3. This workbook contains multiple worksheets that are stratied by AR Bank BIT sets specically
curated to validate updated CLSI breakpoints for specic antimicrobials (Table 1). Each worksheet is
prelled with the list of curated isolates and their AR Bank minimal inhibitory concentration (MIC)
values and interpretations.
a. BIT-1 (AR Bank panel name: BIT): Enterobacterales for ertapenem, meropenem, imipenem,
cefazolin, aztreonam, cefepime, ceftazidime, cefotaxime, ceftriaxone, ciprooxacin, or
levooxacin.
b. BIT-2 (AR Bank panel name: PTA): Enterobacterales for piperacillin/tazobactam, gentamicin,
tobramycin, amikacin, or colistin.
c. BIT-3 (AR Bank panel name: PFA): Pseudomonas aeruginosa for meropenem, imipenem,
aztreonam, cefepime, ceftazidime, piperacillin/tazobactam, tobramycin, ciprooxacin,
levooxacin, or colistin.
d. BIT-4 (AR Bank panel name: ACI): Acinetobacter spp. for meropenem, imipenem, or colistin.
NOTE: Within each BIT worksheet, the specic antimicrobials are independent of each other. This
means the user has the choice to validate or verify some, but not all, antimicrobials.

4
Accuracy Instructions for Prelled Excel
Table 1. Summary of Organism and Breakpoints Included in the Breakpoint Implementation Toolkit
Antimicrobial Reporting Group
2023 CLSI Breakpoint
(S/I or SDD/R) BIT Set
Amikacin Enterobacterales 4/8/16 BIT-2
Aztreonam Enterobacterales 4/8/16 BIT-1
Pseudomonas aeruginosa 8/16/32 BIT-3
Cefazolin Enterobacterales 2/4/8 BIT-1
Cefepime Enterobacterales 2/4-8/16 BIT-1
Pseudomonas aeruginosa 8/16/32 BIT-3
Cefotaxime Enterobacterales 1/2/4 BIT-1
Ceftazidime Enterobacterales 4/8/16 BIT-1
Pseudomonas aeruginosa 8/16/32 BIT-3
Ceftriaxone Enterobacterales 1/2/4 BIT-1
Colistin Enterobacterales 2/4 BIT-2
Pseudomonas aeruginosa 2/4 BIT-3
Acinetobacter baumannii 2/4 BIT-4
Ciprooxacin Enterobacterales 0.25/0.5/1 BIT-1
Pseudomonas aeruginosa 0.5/1/2 BIT-3
Ertapenem Enterobacterales 0.5/1/2 BIT-1
Gentamicin Enterobacterales 2/4/8 BIT-2
Imipenem Enterobacterales 1/2/4 BIT-1
Pseudomonas aeruginosa 2/4/8 BIT-3
Acinetobacter baumannii 2/4/8 BIT-4
Levooxacin Enterobacterales 0.5/1/2 BIT-1
Pseudomonas aeruginosa 1/2/4 BIT-3
Meropenem Enterobacterales 1/2/4 BIT-1
Pseudomonas aeruginosa 2/4/8 BIT-3
Acinetobacter baumannii 2/4/8 BIT-4
Piperacillin/tazobactam Enterobacterales 8/16/32 BIT-2
Pseudomonas aeruginosa 16/32/64 BIT-3
Tobramycin Enterobacterales 2/4/8 BIT-2
Pseudomonas aeruginosa 1/2/4 BIT-3
Abbreviations: BIT, breakpoint implementation toolkit; I, intermediate; R, resistant; S, susceptible.

5
Accuracy Instructions for Prelled Excel
4. For each BIT set, 5 isolates have been suggested for reproducibility/precision testing. Within
each BIT set tab, these recommended isolates are highlighted in green. These isolates are
recommendations; the laboratory may decide to use other strains, including QC organisms. At
minimum to evaluate precision; 3 isolates should be included for each antimicrobial agent.
5. Data are only entered in cells that are shaded orange (see below for detailed instructions). Other
cells contain formulas that depend upon data entered and must not be modied. There are also
hidden cells that perform modications and/or calculations to the data that are necessary for
proper evaluation.
6. The worksheets are protected for the user’s benet. However, if a user would like to view hidden
columns and/or view/edit formulas, the worksheets can be unprotected without a password.
7. Users should not copy and paste data from external sources to this spreadsheet, doing so
invalidates data validation rules set to certain cells. Mathematical calculations in hidden cells
depend upon proper data format (ie, all numeric values for MICs).
8. The Summary tab will display aggregate accuracy performance from each completed study
by antimicrobial and reporting group. If multiple reporting groups were tested for a single
antimicrobial, the combined overall accuracy is displayed.
WARNING: Any manipulation beyond the instructions provided here may invalidate formulas and
lead to erroneous results.

6
Accuracy Instructions for Prelled Excel
Accuracy
Accuracy is dened as the closeness of agreement between a measured quantity value and a true quantity
value of a measurand (AR Bank MIC). For AST, accuracy is the agreement of the test system result with the
result generated for the same isolate with the appropriate reference method. To establish the performance
characteristics of a test system, a minimum of 30 isolates should be tested. If a laboratory were verifying
the performance characteristics established by the manufacturer, the lab may choose to test fewer isolates.
Final results should be used if discrepancy testing was performed.
Using the Excel for Accuracy
1. Find the appropriate BIT tab and navigate horizontally to the specic antimicrobial being validated.
This area is indicated by a header describing drug name and CLSI breakpoints being validated.
Ertapenem is shown below.

7
Accuracy Instructions for Prelled Excel
2. Only cells that are orange are editable. For test data, only an MIC result is needed; the
interpretation is automatically generated based on the MIC entered.
3. For each isolate, enter the results (MIC and sign associated with MICs for off-scale values) obtained
from the test system/method being evaluated in the [Insert Antimicrobial] Test Data columns. The
signs “<=” is used in place of “≤”, and “≥ ” is used in place of “≥”. Data validation rules exist for both
the Test Sign and Test MIC columns. Users may use the dropdown function (see below) or type in
the character or values. If users type in a character or value not in the list, the entry will be rejected.
a. Enter the numerical MIC value in the Test MIC column.
i. When testing a combination agent (eg, piperacillin/tazobactam), only enter the value for
the rst component (eg, piperacillin).
ii. When entering data generated by gradient diffusion strips, round up any “in-between” MIC
values to the next higher log
2
dilution (eg, 1.5 is rounded up to 2).
iii. For lower MICs, the actual concentration should be used (ie, enter 0.12 as 0.125, 0.06 as
0.063, 0.03 as 0.031…). NOTE: This is specic to this workbook and not related to reporting
of MICs. This format is necessary to accurately calculate essential agreement after the MIC
conversion to inverse of logarithmic base 2 function: log
2
(MIC)=x, where 2
x
=MIC.
b. For MIC results that are on-scale (eg, =8), leave Test Sign column blank. See AR Bank isolate #1
below.
c. For MIC results that are off-scale, enter the appropriate sign in the Test Sign column (eg, if the
result is >16, enter or select “>”). See AR Bank isolate # 2 below.
d. There is no need to calculate the categorical interpretation. The interpretation is automatically
generated based upon the MIC entered.

8
Accuracy Instructions for Prelled Excel
WARNINGS: Do not use default copy/paste function or drag data from another excel worksheet or
cell. This action will replace existing data validation rules with validation rules from the source and
may invalidate formulas, leading to erroneous calculations. Copy and paste function may be used
if using the Paste Values under Paste Options. Proceed as follows: select the range of cells to copy
data into and right click; select the arrow at Paste Special; select the rst option under Paste Values.
Do not copy/paste or drag data from one cell to another in the same worksheet across worksheets.
This action may lead to changes in cell reference that are precedents to certain calculations in
hidden columns and can lead to erroneous calculations.
Clearing Test Data
If Macros are disabled: Highlight the test data cells to be cleared (Test Sign and/or Test MIC), right click and
select “Clear Contents.” This may need to be done in chunks or using the CTRL key.
If Macros are enabled: Click the blue “Clear [Insert Antimicrobial Name] TestData” button (indicated by
the arrow below) and conrm you want to proceed with deleting test results. This will clear the Test Sign
and Test MIC columns. Each antimicrobial agent has a specic clear button.
NOTE: The use of Macros clears the Excel “undo stack”. This means you can no longer undo (CTRL+Z) to
the previous step or action. Saving the le prior to use on a cloud-based le system with version control is
recommended. This allows recovery of previous versions of the workbook if data is accidentally cleared by
the macro.

9
Accuracy Instructions for Prelled Excel
Analysis Within the BIT Set Worksheets
As test data are entered, analysis of the results will ensue under the “Calculations” section. This area is
read-only and cannot be edited. These columns compare the Test MICs and categorical interpretation (Test
Interp) from the test system to the AR Bank MIC and categorical interpretation results (Interp).
Essential Agreement (EA): MIC result obtained with the AST system that is within one doubling dilution
step from the AR Bank MIC value. Calculations are scaled and adjusted for off-scale results (ie, >32 and
>64 are within EA; >32 and 32 are within EA; >32 and 16 are not within EA). If the Test MIC of the isolate
is in essential agreement with the AR Bank MIC, the eld will display YES; if the results are not in essential
agreement, the eld will display NO and be highlighted red. The overall EA is expressed as a percentage of
those data points within EA divided by the total number of isolates tested.
Dilutions: This is the calculated number of log
2
dilutions the Test MIC deviates from the AR Bank MIC. This
is scaled to the most conservative MIC and adjusted for off-scale results (ie, >32 and >8= 0[exact]; >32 and
32 = -1; >32 and >=32 = 0). This column can be used to assess overall bias of results.
Category Agreement (CA): The agreement of interpretation (eg, susceptible, intermediate, susceptible-dose
dependent, nonsusceptible, and resistant) results between the test and the interpretation of the AR Bank
MIC result. If the Test Interp of the isolate is in agreement with the AR Bank Interp, the eld will display
YES; if the results are not in categorical agreement, the eld will display NO and be highlighted red.
The overall CA is expressed as a percentage of those data points within CA divided by the total number of
isolates tested.
Errors: The type of error (minor, major, very major) if CA is not obtained.
• Minor Error (minE): Difference in Test Interp and AR Bank Interp where one result is intermediate (I)
(or susceptible-dose dependent [SDD]) and the other is susceptible or resistant. The overall minor
error frequency is expressed as a percentage of those data points having a minor error divided by the
total number of isolates tested.
• Major Error (ME): Error when the AR Bank Interp is susceptible and the Test Interp is resistant. The
overall major error frequency is expressed as a percentage of those data points having a major error
divided by the total number of susceptible AR Bank isolates tested.
• Very Major Error (VME): Error when the AR Bank Interp is resistant and the Test Interp is susceptible.
The overall very major error frequency is expressed as a percentage of those data points having a very
major error divided by the total number of resistant AR Bank isolates tested.

10
Accuracy Instructions for Prelled Excel
Precision/Reproducibility
Each AR Bank BIT set includes a corresponding Precision tab. Precision (or reproducibility) is dened as
closeness of agreement between the results of successive measurements of the same analyte. Up to ve
isolates may be tested three separate times (separate inoculum preparations) to characterize the precision
of the test system. These isolates may include a combination of AR Bank isolates and quality control strains
(eg, ATCC strains).
Using the Excel for Precision (Reproducibility)
1. Navigate to the appropriate precision tab and antimicrobial section. Each BIT set has a
corresponding precision tab. Within the tab, each drug has a separate table for calculating
precision. The aztreonam table is shown below. Like the accuracy tabs, cells that are orange are
editable.
2. Enter the Isolate ID and organism.
3. For each isolate, enter the categorical result for each replicate (Result 1, Result 2, Result 3) and the
date and initials of the tester.
4. Once the precision data is entered for each isolate, calculations will proceed in gray columns of the
table. The most commonly occurring (mode) category is calculated. If no mode exists, then middle
interpretation (or “median”) is used in the rank of S, I or SDD, R.
a. If R, R, and I replicate categorical results were obtained for an isolate, the “R” interpretation is
considered the mode.
b. If S, I, R replicate categorical results were obtained for an isolate, the “I” interpretation is
considered the middle.
5. Each result is compared to the modal or middle categorical interpretation. The results are
considered equivalent if the interpretation of the test result and modal or middle interpretation are
the same.
6. The overall category equivalency is calculated as the number of equivalent data points divided by
the total number of data points.

11
Accuracy Instructions for Prelled Excel
Reviewing Aggregated Summary Data
Data are gathered from each tab and antimicrobial/organism validation or verication study to a summary
table. The table can display the studies’ characteristics (such as number of total isolates tested, number of
susceptible and resistant strains) and overall descriptive statistical calculations. The hyperlinks will navigate
back to the corresponding antimicrobial’s accuracy tab within the BIT worksheet.
The table adds an additional element of aggregate data for combined reporting groups. The “Combined”
under “Reporting Group” is calculated when multiple reporting groups are tested for a single antimicrobial.
Overall calculations are performed combining all isolates for all reporting groups. See below for combined
analysis of meropenem.

12
Accuracy Instructions for Prelled Excel
NOTE: Essential agreement is not displayed, but can be unhidden, as breakpoint update validation and
verication studies should focus assessment of performance on the ability to accurately assign the
interpretation based on the new breakpoints.
13
Accuracy Instructions for Prelled Excel
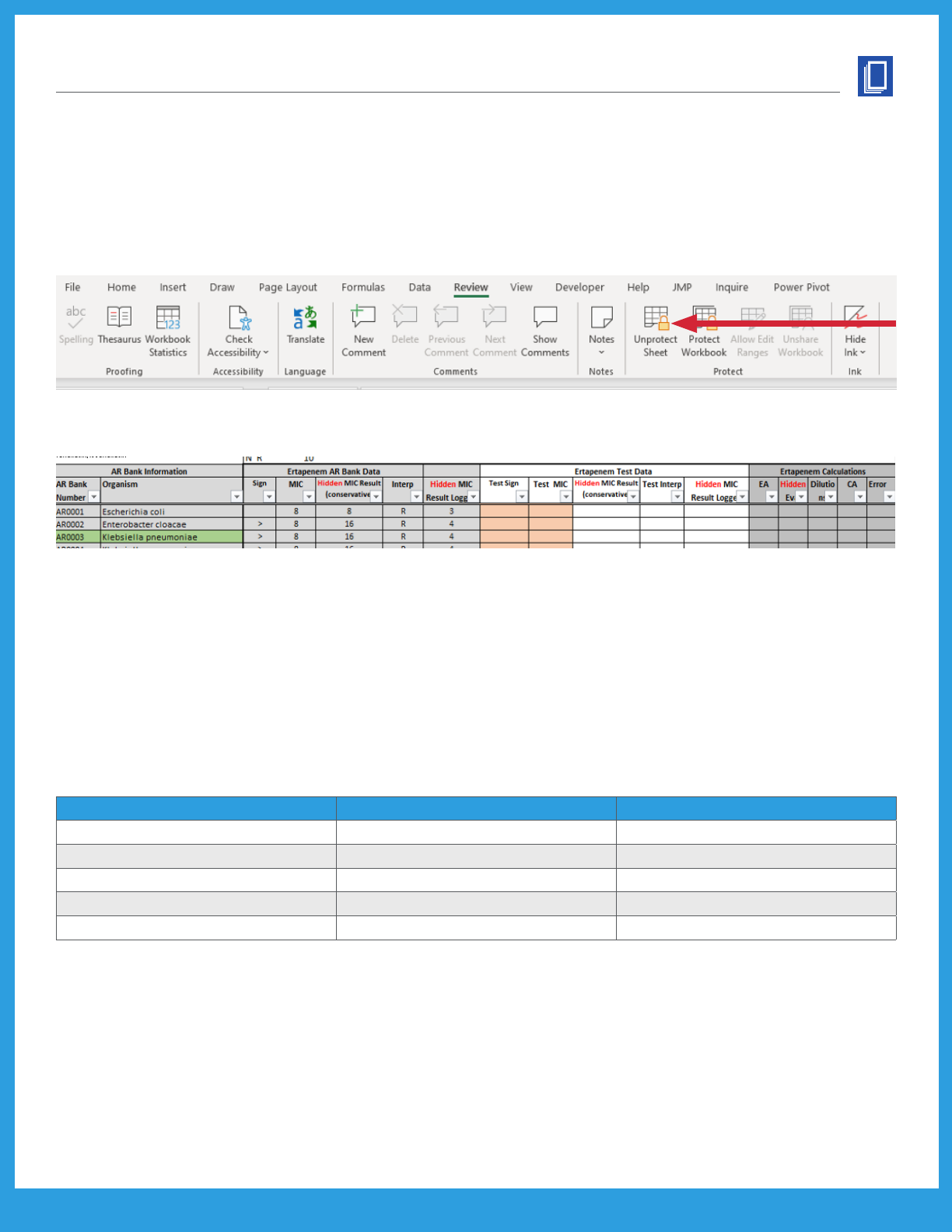
Unlocking the Spreadsheet and Hidden Columns
Each sheet in the workbook is protected, except for the cells used for user data entry. This is done to
prevent user error that may affect calculation cells (eg, misclicks). Users can unlock the sheets without a
password as needed. To unlock the specic sheet, navigate to the Review ribbon in the menu bar, and click
“Unprotect Sheet.” There is no password needed. Any changes should be performed only by experienced
Microsoft Excel users.
There are ve hidden columns that perform intermediary calculations necessary to determine EA, number
of Dilutions from the AR Bank MIC, CA, and Error type.
The logic for the hidden columns is described below:
• Hidden MIC Result Conservative (both AR Bank and Test Data areas) - Incorporates the sign and
calculates the most conservative MIC. This only affects MICs with greater than sign (>), which bumps
the MIC to the next higher dilution. For devices that report a greater than or equal to sign (>=, ≥)
or less than or equal to sign (<=, ≤), the most conservative MIC is the MIC reported with the sign.
Examples of the conversions with and without the various signs are in the table below.
• Hidden MIC Result Logged - MIC conversion to inverse of logarithmic base 2 function: log
2
(MIC)=x,
where 2
x
=MIC This allows EA and dilution difference to be calculated. Examples of the logged
conversions are below.
• Hidden Eval - This variable indicates whether an MIC pair is evaluable with a “Yes” or “No” value.
Evaluable is dened as when either reference or test MIC is on scale and that can be determined with
certainty to be at least +1 or -1 dilution from each other along the combined scaled concentration
range of MICs (it takes into account when MIC dilutions are not the same and scales it to the most
conservative). This is an informational variable and not currently used in any calculations.
Reported MIC Converted MIC Conservative Result MIC Result Logged
16 16 4
> 16 32 5
>=16 16 4
0.5 0.5 -1
<=0.5 0.5 -1

